Untangling the Complexities of Cell and Gene Therapy Clinical Trials: A Supply Chain Perspective
Pharmaceutical Technology
MAY 24, 2023
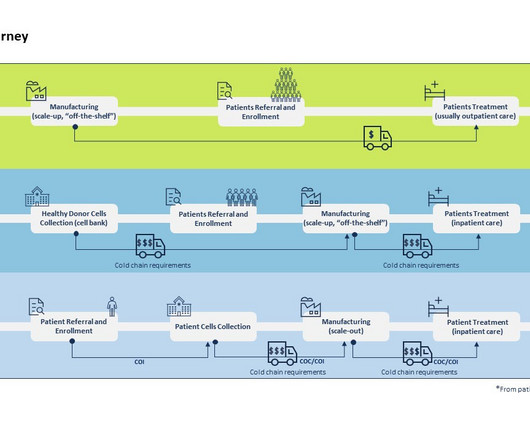
CGT have highly unique and specialized manufacturing processes. Cold chain requirements for CGT are highly stringent and most often at the cryogenic level (below -150 Celsius), which requires the use of specialized containers filled with liquid nitrogen (dewars) and particular shippers requiring specific logistics expertise.





















Let's personalize your content