The power of rapid methods for fungal ID
European Pharmaceutical Review
JULY 10, 2023
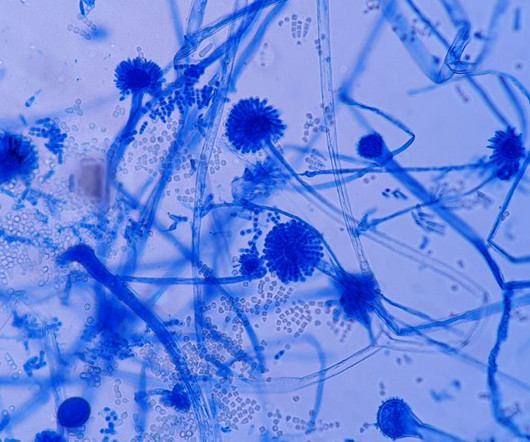
Fungal contamination in pharmaceutical products represents a potential hazard for two reasons. First, it may cause product spoilage; the metabolic versatility of fungi is such that any formulation ingredient may undergo chemical modification in the presence of a contaminating organism.





































Let's personalize your content