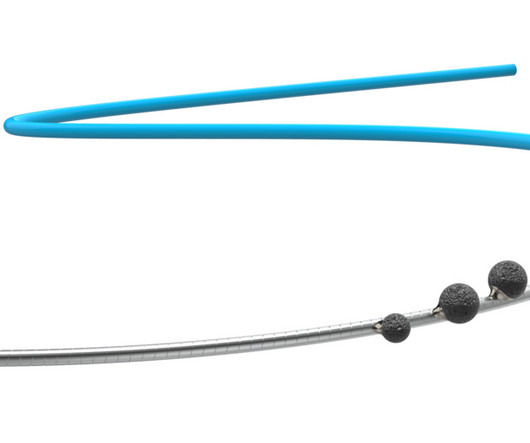
Cardio Flow, Inc., Announces U.S. Food and Drug Administration (FDA) 510(k) Clearance for its FreedomFlow® Orbital Atherectomy Peripheral Platform
Legacy MEDSearch
OCTOBER 18, 2023
Food and Drug Administration (FDA) 510(k) clearance for the company’s FreedomFlow Orbital Atherectomy Peripheral Platform. CEO of Cardio Flow, stated: “Cardio Flow is committed to providing meaningful solutions that directly address the needs of physicians and their PAD patients through innovative product development.
































Let's personalize your content