FDA approves Roche, Novartis' Xolair to prevent severe outcomes from common food allergies
Fierce Pharma
FEBRUARY 16, 2024
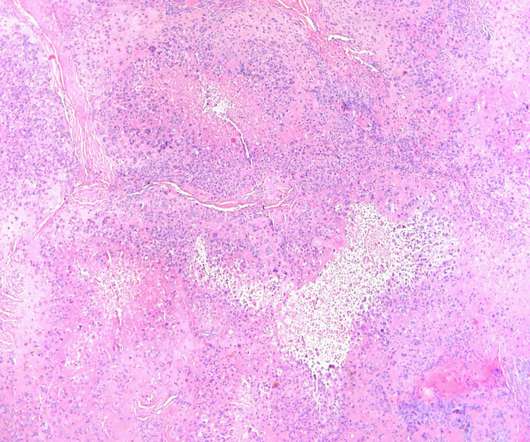
People with food allergies finally have a drug that can help prevent severe outcomes—and it’s a drug that’s been on the market for two decades. People with food allergies finally have a drug that can help prevent severe outcomes—and it’s a drug that’s been on the market for two decades.








































Let's personalize your content