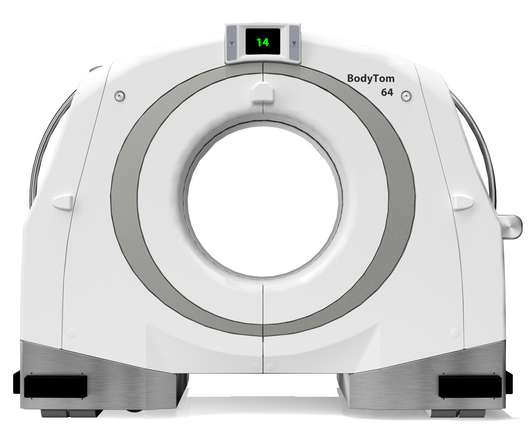
NeuroLogica Announces FDA 510(k) Clearance of BodyTom® 64
Legacy MEDSearch
NOVEMBER 28, 2022
a subsidiary of Samsung Electronics Co. Food and Drug Administration for commercial use in the United States. Director of Global Sales and Marketing of NeuroLogica. the healthcare subsidiary of Samsung Electronics Co., NeuroLogica Corp., About NeuroLogica. NeuroLogica Corp.,









Let's personalize your content