The ingredients of a successful biopharma collaboration
PharmaVoice
JUNE 13, 2023
Collaboration is important along the entire spectrum and lifecycle of biopharma, and finding an effective partner is harder than it sounds.

PharmaVoice
JUNE 13, 2023
Collaboration is important along the entire spectrum and lifecycle of biopharma, and finding an effective partner is harder than it sounds.

MedReps
JUNE 13, 2023
Communication is important, especially between medical sales reps and sales team leaders. In order for everyone to be on the same page, as well as deal with successes and setbacks accurately, there needs to be open communication. If your medical sales reps are too afraid to talk to you about their issues with the job, such as problems making a certain sale or the inability to find a certain client’s pain point, then they will more than likely not meet their quotas and start looking to find anoth
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

PharmaVoice
JUNE 14, 2023
The big pharma patent cliff is almost here — and could change the face of the industry. Here’s a look at the key stats behind the coming wave of blockbuster losses.

Fierce Pharma
JUNE 12, 2023
Two years after the initial COVID-19 vaccine push swept across the globe, Pfizer’s COVID-19 vaccine partner BioNTech is heading to court in its home country of Germany to defend itself against alle | The drugmaker will defend itself against claims from a German healthcare worker who sued the company for at least 150,000 euros ($161,500). The plaintiff alleges she suffered bodily harm resulting from Pfizer and BioNTech's Comirnaty vaccine.

Advertisement
What if you could help your sellers stop wasting 72% of their day on non-selling activities and focus on bringing in revenue? Incorporating AI in your enablement workflows can help you cut down on busy work, get projects done faster, and let your team (and you!) focus on making a bigger impact. We put together this guide to show you how to use AI to cut time and costs for projects, including collateral creation, development of training videos, and automating tedious processes.
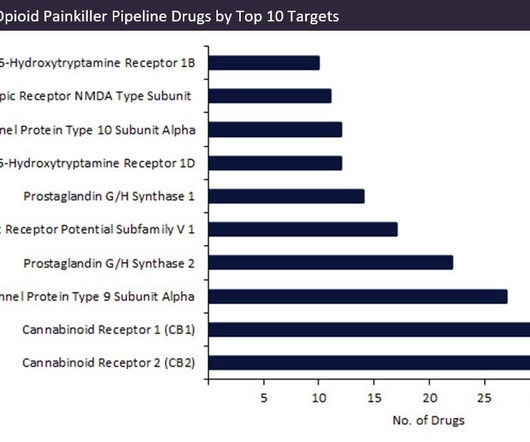
Pharmaceutical Technology
JUNE 12, 2023
On 9 May, National Fentanyl Awareness Day was observed in recognition of the US opioid crisis. Accordingly, the development of non-opioid painkillers is experiencing a surge in activity and despite numerous novel targets receiving high levels of attention, cannabinoids have emerged as strong favourites to replace opioid-related medications. The North American opioid epidemic highlights limitations in opioid use, such as the potential for drug abuse and overdose.

MedCity News
JUNE 14, 2023
Hospitals’ digital health adoption exploded during the pandemic, leading to many vendor contracts spanning three to five years. As these contracts reach their expiration dates over this year and next, a new report predicts that telemedicine platforms and remote patient monitoring tools face the highest risk of being turned over by hospitals.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

Fierce Pharma
JUNE 12, 2023
Bayer recently laid out its ambition | Bayer recently laid out its ambition to achieve $10 billion in sales from its oncology business by 2030 and become a top 10 cancer drug player. To get there, the company is looking outside for a “midsize acquisition,” Bayer’s oncology chief Christine Roth said.

PharmaTimes
JUNE 12, 2023
The treatment candidate, known as MDI-26478, is a positive allosteric modulator of the AMPA receptor - News - PharmaTimes

MedCity News
JUNE 16, 2023
To increase your return on technology investment, start with an audit of existing technology. Where is it falling short? Do problems stem from lack of functionality or troubles with adoption? What do all users think?

European Pharmaceutical Review
JUNE 13, 2023
A new therapeutic radionuclide facility, the world’s largest production site of lutetium-177, has opened in Germany. ITM Isotope Technologies Munich SE ( ITM )’s manufacturing plant in Neufahrn near Munich will produce the innovative medical isotope for targeted cancer therapies. “Radiopharmaceuticals are an essential new class of anti-cancer drugs that have the potential to improve therapy outcomes and quality of life for many patients.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Fierce Pharma
JUNE 16, 2023
Merck’s cancer star Keytruda could be on its way to an updated label in HER2-positive stomach cancer after showing it can stave off tumor progression in a combination study. | After scoring accelerated approval to treat HER2-positive stomach cancer in 2021, the drug has now shown it can stave off tumor progression in patients with PD-L1 positive tumors.
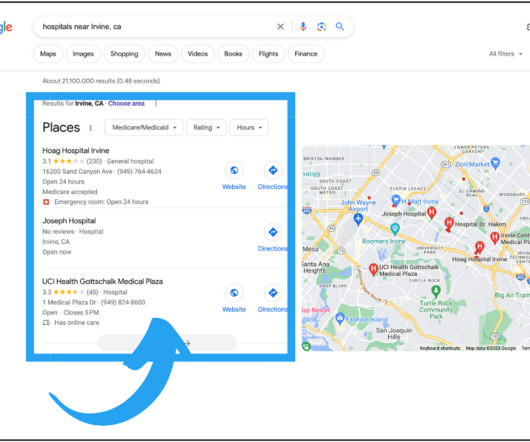
Healthcare Success
JUNE 12, 2023
Consumers across the globe have come to rely on search engines for reliable, trustworthy, accurate, and timely healthcare information. This has significantly increased competition among providers, making SEO—particularly local SEO—a crucial part of any digital marketing strategy. Local SEO is beneficial for any size business but critical for multilocation companies.

MedCity News
JUNE 16, 2023
The consensus is 2022 was a challenging year for digital health companies. Venture capital and other startup investment in the U.S. digital health sector plummeted to $15 billion from more than $29 billion in 2021, according to Rock Health. Market analysts CB Insights similarly tracked private investment last year in U.S. digital health at $17.7 billion in 2022 — down 56% from $40.2 billion in 2021.

European Pharmaceutical Review
JUNE 11, 2023
The US Food and Drug Administration (FDA) has approved commercial production at Bristol Myers Squibb’s newest cell therapy manufacturing facility in Devens, Massachusetts. The 244,000 square foot facility in Devens is BMS’ third commercial cell therapy facility in the US. It is located on the company’s existing Devens site, which has been developing, producing, and testing clinical and commercial medicines for over a decade.

Fierce Pharma
JUNE 16, 2023
In the battle for superiority in the field of next-gen diabetes and obesity treatments, Novo Nordisk holds the lead as the developer of the metabolism-regulating treatment semaglutide. | In the battle for diabetes and obesity superiority, Novo Nordisk holds a head start as the original developer of the metabolism-regulating treatment semaglutide. But Eli Lilly is quickly gaining ground and is primed to become the market leader with its GLP-1 treatment Mounjaro, according to GlobalData.

PharmaTimes
JUNE 13, 2023
525-patient trial intends to assess ‘complement activation’ during HD in patients with end stage renal failure - News - PharmaTimes

MedCity News
JUNE 11, 2023
The Making Care Primary Model will be tested by the Center for Medicare and Medicaid Innovation from July 1, 2024, to December 31, 2034, in Colorado, Massachusetts, Minnesota, New Jersey, New Mexico, New York, North Carolina and Washington.
European Pharmaceutical Review
JUNE 13, 2023
If approved, VLA1553 could become the first licensed chikungunya vaccine” A single vaccination of VLA1553 has been shown to produce neutralising antibody levels, thought to protect against chikungunya disease in 99 percent of Phase III trial participants. The chikungunya vaccine candidate is the only one globally for which regulatory review processes are underway.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Fierce Pharma
JUNE 16, 2023
Less than a month after AbbVie and Genmab won FDA approval for Epkinly, Roche has crossed the finish line with its bispecific answer to large B-cell lymphoma. | Less than a month after AbbVie and Genmab won FDA approval for Epkinly, Roche has crossed the finish line with its bispecific answer to large B-cell lymphoma, though with a narrower label.

PM360
JUNE 16, 2023
Funding and access are not new topics of conversation in the rare disease space. Genzyme made headlines in the ’90s when it launched the enzyme replacement therapy Cerezyme for a then-unprecedented $300,000 per year. In 2007, Alexion set a new pricing milestone of $500,000 annually for Soliris , a treatment for an ultra-rare blood disorder. As prices continued to rise, industry analysts began to publish top 10 lists cataloging extremely expensive rare disease brands.

MedCity News
JUNE 12, 2023
he FDA has made a purposeful choice to write in broad strokes, stopping short of detailing ways to execute DCTs. Even so, the Agency is starting to acknowledge – and thereby support – the global shift towards expanded trial models. Essentially, the FDA is implicitly broadening the definition of clinical trials.
European Pharmaceutical Review
JUNE 15, 2023
A paper published in the Journal of Pharmaceutical Research International has reviewed microbiological testing for medical devices and described their applications and challenges. Microbiological testing is essential for quality control , regulatory compliance and can help minimise the risk of microbial contamination. This approach can also help determine the appropriate manufacturing processes, sterilisation methods, and maintenance procedures.

Advertisement
Every marketer knows how important it is to prove their efforts drive sales opportunities, but that’s easier said than done. When problems like sales and marketing misalignment, lack of data, and wasted efforts persist, marketers can’t measure, prove, or increase their impact on revenue at a time when demonstrating marketing value is critical. Using analyst and expert data, this guide to marketing impact and content attribution explains: How B2B buyers use content The most common types of conten

Fierce Pharma
JUNE 14, 2023
Sanofi is jumping on the artificial intelligence boat with a new app and a pledge to become “the first pharma company powered by artificial intelligence at scale.” | Sanofi's new app, plai, provides teams with a “360° view" to aid decision-making. The platform is one step in the company's digital transformation and goal to become the "first pharma powered by AI at scale.

PM360
JUNE 16, 2023
While all patient communities and advocacy groups offer patients, their families, and their caregivers support, advice, and information, rare disease patient groups can be especially tight-knit. With such limited information out there for some rare diseases, these groups are often the only resource for people looking for answers. Furthermore, these patients are typically the ones raising money for research into potential treatments and cures, as well as banding together to try to change governme

MedCity News
JUNE 13, 2023
Many health systems aren’t employing the right tactics for hiring and retaining nurses, according to a new report. It argued that hospitals would have an easier time hiring and retaining nurses if they focused more on the things workers want most from their employers — such as flexible scheduling and professional development opportunities.

Pharmaceutical Technology
JUNE 16, 2023
France-based biotech company Osivax has thrown its hat into the influenza ring by dosing the first subject with its vaccine candidate OVX836. The Phase IIa trial (NCT05734040) in Australia will see a potential 500 volunteers given OVX836 in combination with quadrivalent influenza vaccines (QIVs). The developer has tested OVX836 in four completed clinical trials.

Advertiser: ZoomInfo
Marketing technology is essential for B2B marketers to stay competitive in a rapidly changing digital landscape — and with 53% of marketers experiencing legacy technology issues and limitations, they’re researching innovations to expand and refine their technology stacks. To help practitioners keep up with the rapidly evolving martech landscape, this special report will discuss: How practitioners are integrating technologies and systems to encourage information-sharing between departments and pr

Fierce Pharma
JUNE 12, 2023
Another biosimilar product copying Johnson & Johnson’s top-selling drug Stelara may enter the U.S. market without a patent infringement challenge by early 2025 thanks to a new settlement. | Following a deal with Amgen, J&J has granted Alvotech and Teva a license to launch their Stelara biosimilar no later than Feb. 21, 2025.

European Pharmaceutical Review
JUNE 16, 2023
According to a recent review funded by GSK Biologicals, artificial intelligence (AI) and machine learning (ML) seems intuitively, to be well suited to perform pharmacovigilance (PV) tasks given the large volume of data, high degree of uncertainty and need to learn from data. However, the paper highlighted the challenges these technologies hold for analytics.

MedCity News
JUNE 13, 2023
Ipsen drug Bylvay is now FDA approved for treating pruritus, or severe itching, which is a complication of the rare liver disease Alagille syndrome. The oral drug was previously approved for treating pruritus in another rare inherited liver disease called PFIC.

PM360
JUNE 16, 2023
According to the National Organization for Rare Disorders (NORD) , there are over 7,000 known rare diseases, with 90% having no effective treatment method or cure. 1 When an individual is diagnosed with a rare disease, they often experience fear, uncertainty, and anxiety. Obtaining a diagnosis is only the first step, leaving many without the direction or resources to find the additional medical care they need.
Let's personalize your content