Kansas AG accuses Pfizer of misrepresenting COVID vaccine, hiding safety risks in lawsuit
Fierce Pharma
JUNE 18, 2024
Kansas Attorney General is accusing Pfizer of "misrepresenting" its vaccine's safety and efficacy in a 179-page civil suit.
Fierce Pharma
JUNE 18, 2024
Kansas Attorney General is accusing Pfizer of "misrepresenting" its vaccine's safety and efficacy in a 179-page civil suit.

MedCity News
JUNE 18, 2024
Talkiatry’s funding round was led by Andreessen Horowitz. With the financing, the company will bring on more providers and advance value-based care. The post Talkiatry Raises $130M To Expand Behavioral Health Access appeared first on MedCity News.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Fierce Pharma
JUNE 18, 2024
While AstraZeneca’s Truqap bears the distinction of being the first AKT inhibitor to pass muster with the U.S. | In the late-stage CAPItello-290 study, Truqap plus the chemotherapy paclitaxel failed to help extend the lives of patients with locally advanced or metastatic triple-negative breast cancer (TNBC) over paclitaxel and placebo alone, AstraZeneca said Tuesday.
MedCity News
JUNE 18, 2024
Day One Biopharmaceuticals is adding an antibody drug conjugate to its pipeline, licensing rights to a molecule from MabCare Therapeutics. The MabCare ADC addresses PTK7, a protein found in many types of solid tumors. The post Cancer Biotech Day One Biopharma Expands to ADCs, Paying $55M to Nab a Phase 1-Ready Drug appeared first on MedCity News.

Advertisement
What if you could help your sellers stop wasting 72% of their day on non-selling activities and focus on bringing in revenue? Incorporating AI in your enablement workflows can help you cut down on busy work, get projects done faster, and let your team (and you!) focus on making a bigger impact. We put together this guide to show you how to use AI to cut time and costs for projects, including collateral creation, development of training videos, and automating tedious processes.

Fierce Pharma
JUNE 18, 2024
Intra-Cellular Therapies’ surging antipsychotic Caplyta has scored again with remarkable trial results, fueling its bid for a third and potentially most lucrative indication—major depressive disord | Intra-Cellular Therapies’ surging antipsychotic Caplyta has scored again with remarkable trial results, fueling its bid for its third and potentially most lucrative indication—major depressive disorder.

MedCity News
JUNE 18, 2024
Ransomware group Black Basta has quickly gained prominence as one of the biggest threats to healthcare providers’ cybersecurity. The group, which is responsible for the Ascension cyberattack, has hit more than 500 organizations across the globe since it first appeared in April 2022. The post 3 Things to Know About the Cybergang that Attacked Ascension appeared first on MedCity News.
Pharma Rep Focus brings together the best content for pharma rep professionals from the widest variety of industry thought leaders.

PharmaVoice
JUNE 18, 2024
Drug launches have underperformed expectations at a high rate, and pharmas need to get better at thinning the pipeline to make room for the real wins, says a life sciences consultant.
Fierce Pharma
JUNE 18, 2024
Authors: Arlene Pineda, Senior Director, Patient Support Services, IQVIA Kristina Jones, Principal, Patient Services Technology & Analytics, IQVIA | In a curious twist, patient support programs — often designed to alleviate the burden on patients — sometimes create unintended challenges. These large, intricate, and costly initiatives can paradoxically complicate the lives of the very individuals they aim to assist.

MedCity News
JUNE 18, 2024
Administrative inefficiencies create a bottleneck that hinders the entire healthcare system. The post Innovation Alone Isn’t Enough: Addressing the Legacy of Administrative Burdens in Healthcare appeared first on MedCity News.

Pharmaceutical Technology
JUNE 18, 2024
Johnson & Johnson has filed a BLA with the US FDA seeking approval for SC amivantamab for the treatment of non-small cell lung cancer.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

MedCity News
JUNE 18, 2024
Health plans should be sure the following elements are incorporated into their genetic testing benefits framework. The post 9 Requirements for an Optimal Genetic Test Benefit Program appeared first on MedCity News.

PharmaTech
JUNE 18, 2024
The research collaboration and licensing agreement will focus on the discovery and development of RNA exon editing therapeutics.

pharmaphorum
JUNE 18, 2024
Novo Nordisk CEO agrees to appear at a Senate hearing to defend the pricing of Ozempic and Wegovy in the US.

PharmaTimes
JUNE 18, 2024
The progressive neurological disorder currently affects more than 200,000 people worldwide

Advertisement
Every marketer knows how important it is to prove their efforts drive sales opportunities, but that’s easier said than done. When problems like sales and marketing misalignment, lack of data, and wasted efforts persist, marketers can’t measure, prove, or increase their impact on revenue at a time when demonstrating marketing value is critical. Using analyst and expert data, this guide to marketing impact and content attribution explains: How B2B buyers use content The most common types of conten

pharmaphorum
JUNE 18, 2024
The rivalry between Pfizer and MSD in pneumococcal vaccines has dialled up a notch with the FDA approval of MSD’s new shot Capvaxive, the first to be aimed specifically at adults.The 21-valent product – formerly known as V116 – covers a wider range of Streptococcus pneumoniae serotypes than any of MSD’s earlier vaccines and eight that are not covered by any other approved pneumococcal vaccine.

Pharmaceutical Technology
JUNE 18, 2024
As Asda sets up a nationwide retail pharmacy in the UK and US grocer pharmacies expand, scaling up this model will be the next frontier.

pharmaphorum
JUNE 18, 2024
KalVista files sebetralstat with the FDA, aiming to make it the first oral, on-demand treatment for rare disease hereditary angioedema (HAE)

Scott Burrows
JUNE 18, 2024
In the dynamic world of business, how we approach challenges can define our path to success. Instead of being bogged down by adversity, we have the power to transform it into opportunity simply by changing the way we ask questions. From “Why” to “What” During tough times, it’s natural to ask ourselves, “Why is this happening to us?

pharmaphorum
JUNE 18, 2024
AstraZeneca’s AKT inhibitor Truqap was unable to improve overall survival in a phase 3 triple-negative breast cancer (TNBC) trial

Pharmaceutical Technology
JUNE 18, 2024
Since emerging from stealth in December 2023, the company has begun establishing its laboratories in Montreal.

pharmaphorum
JUNE 18, 2024
Roche’s move into the digital pathology category has been boosted by FDA approval of a lab instrument and toolkit for use as an aid to clinical diagnosis.The clearance covers Roche’s Ventana DP 200 slide scanner, digital pathology workflow software, and a display that can be used to review and interpret digital images of scanned pathology slides.

Pharmaceutical Technology
JUNE 18, 2024
CRTE-7A2 is under clinical development by Beijing Corregene Biotechnology and currently in Phase I for Anal Cancer.

Advertisement
Quotas need to be hit. Revenue goals need to be met. This reality makes shortening sales onboarding time a top priority. Organizations with a standard onboarding process boost employee retention by 58% and increase productivity by 50%. Unfortunately, many companies struggle with inefficient processes that lead to high turnover and missed revenue opportunities.

pharmaphorum
JUNE 18, 2024
The microbiome is ever more frequently spoken about of late, and so the pharmaphorum podcast invited Leo Grady, founder and CEO of Jona, to speak about at-home personal microbiome profiling, uncovering the relationship between human health and the gut.
Pharmaceutical Technology
JUNE 18, 2024
The US FDA has granted approval for MSD’s KEYTRUDA regimen to treat primary advanced or recurrent endometrial carcinoma in adults.
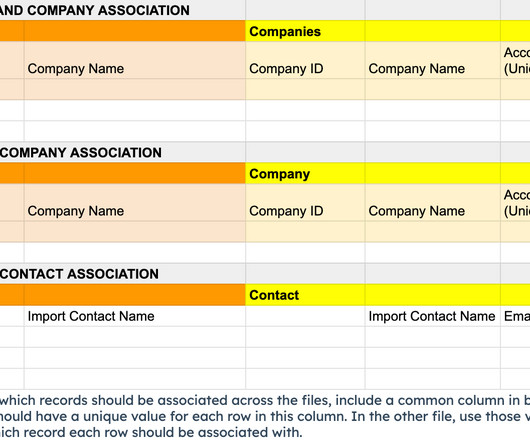
Clear Pivot
JUNE 18, 2024
Learn how to maintain object associations in a CRM migration to HubSpot. Follow a two-stage process to import contacts, accounts, and opportunities seamlessly.

Pharmaceutical Technology
JUNE 18, 2024
The high speed of the transition meant many information technology security teams had insufficient time to install adequate cybersecurity.
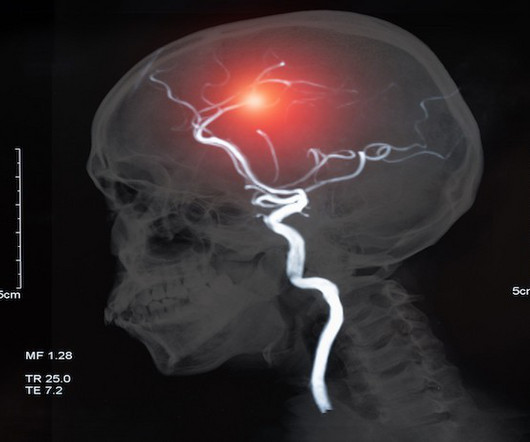
PharmaTimes
JUNE 18, 2024
Stroke causes over seven million new cases annually, ranking second in leading global mortality

MedCity News
JUNE 18, 2024
Three reasons you should hire physicians for your product teams The post Is There a Doctor on the Plane? appeared first on MedCity News.
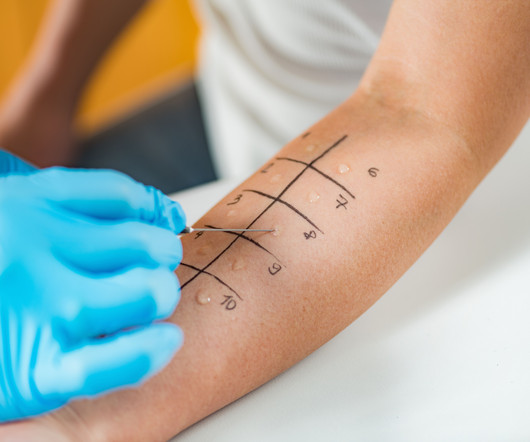
Pharmacy Times
JUNE 18, 2024
The findings also show that skin testing positivity for perennial allergens was higher in patients with childhood-onset asthma compared with those with adult-onset asthma.

PharmaTech
JUNE 18, 2024
QbCon® 1 offers the optimal entry into continuous production. Our truly continuous wet granulator and dryer for research and development guarantees improved product quality while increasing flexibility and operator safety. It also reduces costs by using fewer resources and shortens development cycles by process analytics. Unlike competing systems, QbCon® 1 enables a permanent, truly continuous process without the formation of sub-batches and blocked filters.

Advertisement
Are your sellers unsure about using AI in their day-to-day workflows? Or are they eager to try but uncertain where to start? They might be asking: Which tasks are best suited for AI? How will using AI affect my relationship with my customers? With so many tools available, which ones are the most useful to me? To help answer these and other common questions about using AI during the sales cycle, we surveyed B2B sellers who were early adopters to get their insight and advice about how to use gen A
Let's personalize your content