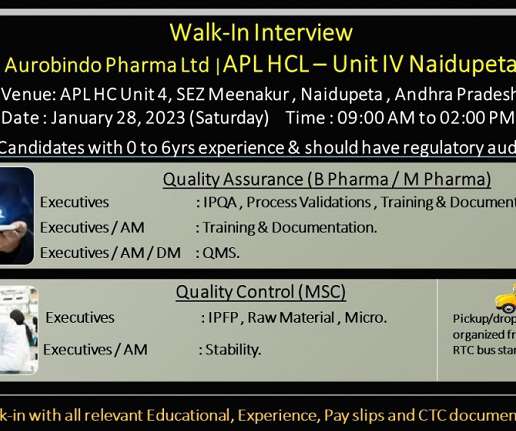
APL Health Care (Aurobindo )-Walk-In Interviews for IPQA/ Microbiology On 4th Feb’ 2023
Pharma Pathway
FEBRUARY 3, 2023
APL is a growing India multinational pharmaceutical manufacturing firm with turnover of over US$2.8 As part of Covid-19 safety measures, candidates are requested to sanitize their hands at the entrance, Maintain social distance and ware face mask before entering the venue. Greetings from Aurobindo !!!





















Let's personalize your content