FDA orders Sun to hire manufacturing consultant for troubled plant as export pause persists
Fierce Pharma
MAY 16, 2023
FDA orders Sun to hire manufacturing consultant for troubled plant as export pause persists fkansteiner Tue, 05/16/2023 - 09:33
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Fierce Pharma
MAY 16, 2023
FDA orders Sun to hire manufacturing consultant for troubled plant as export pause persists fkansteiner Tue, 05/16/2023 - 09:33

Infuse Medical
JULY 4, 2024
If so, it is important to understand the regulatory requirements of the FDA. Do you know the FDA is the Food and Drug Administration? It is essential to understand the FDA’s definition of a medical device. Let’s see FDA regulations for medical device training.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
JULY 21, 2022
Now FDA on-site inspections have resumed, with regulatory authorities returning to physical sites. Throughout the pandemic, the lack of FDA inspections has created new challenges and heightened existing problems, largely due to delays in upgrading legacy facilities. Upgrading facilities. Ensuring GMP readiness.

MedCity News
JULY 27, 2023
In February of 2019, Johnson & Johnson acquired Auris Health and its FDA-cleared Monarch platform for $5.75 Later in 2019, Germany-based Siemens Healthineers announced its acquisition of Corindus and its FDA-cleared CorPath GRX System, a robotics platform specifically for percutaneous coronary interventions.

Pharmaceutical Technology
AUGUST 12, 2024
Leap Consulting Group has launched a practice aimed at guiding Clinical Laboratory Improvement Amendments-certified labs (CLIA) in adhering to the US Food and Drug Administration’s (FDA) Final Rule over the classification of laboratory-developed tests (LDTs).

European Pharmaceutical Review
NOVEMBER 2, 2023
The US Food and Drug Administration ( FDA) has approved Wezlana (ustekinumab-auub) as a biosimilar to Johnson & Johnson’s Stelara (ustekinumab). The evidence also demonstrated that Wezlana met the other legal requirements to be interchangeable with Stelara at the pharmacy level, without consulting the prescriber.

Nixon Gwilt Law
MAY 29, 2024
The FDA regulates all medical devices, including SaMD and DTx, but recognizes that not all pose the same level of risk to patients. For low-risk software devices, the FDA may exercise enforcement discretion, choosing not to enforce certain regulatory requirements. Which SaMD Device Functions May Qualify for Enforcement Discretion?

Rep-Lite
NOVEMBER 30, 2023
B2B sales consulting for medical sales is a specialized service that helps healthcare organizations navigate the unique challenges of B2B transactions within the healthcare industry. What is B2B Sales Consulting? B2B sales consulting involves working with businesses to enhance their sales strategies and operations in a B2B context.

Pharmaceutical Commerce
AUGUST 28, 2024
In an interview with Pharma Commerce Editor Nicholas Saraceno, Jeb Hunter, Senior Regulatory Consultant, EAS Consulting Group, discusses the on “What to Expect When They’re Inspecting: FDA Inspections on DSCSA Compliance" breakout session at the 2024 HDA Traceability Seminar.

PharmaTech
SEPTEMBER 19, 2023
Maik Jornitz, Principal Consultant, BioProcess Resources LLC, discusses the definition of patient safety and how to implement new technologies into upgraded facilities.

World of DTC Marketing
MARCH 8, 2021
Whether it’s through their broker, insurance company, or consultants, businesses should examine these costs closely and understand where they are deviating from benchmarks and why. The FDA could require all pharma product websites to add general health prevention information and links to credible online health information.
Pharma Marketing Network
MARCH 26, 2025
Misinformation and Hallucinations : Generative AI platforms may create content that is inaccurate, outdated, or off-labelviolating FDA regulations. Regulatory Guidance and Gray Areas Currently, guidance from regulatory bodies like the FDA, FTC, and EMA around AI in pharma marketing is limited but growing.
Legacy MEDSearch
OCTOBER 6, 2022
Body Vision Medical, a leader in AI-driven, intraoperative imaging, announced a new partnership with Business Asia Consultants (BAC), Inc. Body Vision Medical’s LungVision system was FDA cleared in 2019 and received CE Mark in 2021. About Business Asia Consultants. Business Asia Consultants (BAC), Inc. Partnership.

Pharma Marketing Network
MARCH 8, 2025
It must convert visitors into leads , provide clear and accurate medical information , and ensure compliance with FDA, HIPAA, and ad platform policies. Avoid misleading claims Always align with FDA-approved language. FDA-Compliant Content & Fair Balance Information Include full prescribing information for branded drug pages.

pharmaphorum
SEPTEMBER 29, 2022
Food and Drug Administration (FDA) regulation as a medical device. Determining whether a product is a medical device subject to FDA regulation necessarily begins with understanding the FDA regulatory definition of ‘medical device’. When assessing mHealth technology, the FDA will determine whether an application is either: i.

Pharma Marketing Network
MARCH 8, 2025
Optimize landing pages to ensure message consistency and compliance with FDA regulations. FDA and HIPAA Compliance in PPC Fair balance requirements Ads must include benefits and risks equally. No unapproved drug claims All claims must be backed by clinical research and FDA-approved labeling. This content is not medical advice.
European Pharmaceutical Review
FEBRUARY 12, 2025
We are pleased that people living with ATTR-CM will have access to another treatment option in the EU, commented Dr Marianna Fontana , PhD, Professor of Cardiology and Honorary Consultant Cardiologist at the National Amyloidosis Centre, Division of Medicine, University College London.
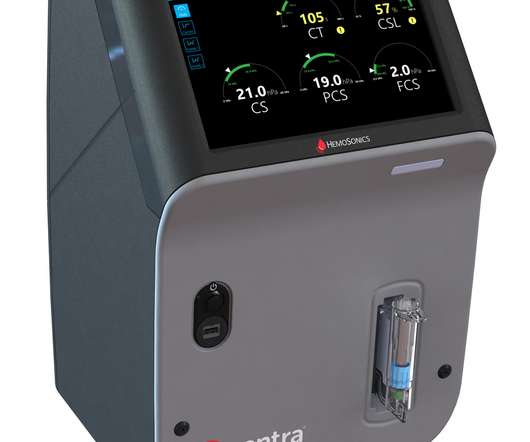
Legacy MEDSearch
DECEMBER 1, 2022
Food and Drug Administration (FDA) for the Quantra Hemostasis System with QStat Cartridge. The FDA clearance of the QStat Cartridge expands the Quantra System’s indications for use to include trauma, and liver transplantation procedures. market today. “Point-of-care data is the answer to PBM-guided patient decisions.

Medical Device Success
MAY 30, 2021
Now he is a consultant for both healthcare systems and some of the largest life science companies in the world. Now he is a consultant for both healthcare systems and some of the largest life science companies in the world. How will the Four Forces shaping healthcare affect your sales and marketing strategies and tactics?

Pharma Marketing Network
MARCH 5, 2025
Unlike traditional businesses, pharma brands face strict advertising regulations , requiring compliance with FDA guidelines, HIPAA, and Googles healthcare ad policies. Strict ad copy guidelines , including FDA-mandated fair balance requirements. Use FDA-approved language and avoid phrases that suggest guaranteed treatment success.

pharmaphorum
JULY 19, 2022
Speaking during a Patients as Partners meeting, Ebony Dashiell-Aje, senior director of patient engagement and outcomes research at BioMarin Pharmaceuticals, said these small, informal, non-regulatory events helped the FDA “better understand what is most important” among specific patient groups. The rise of patient centricity. “We

Pharma Marketing Network
MARCH 20, 2025
Marketers must ensure that their campaigns adhere to FDA, HIPAA, and GDPR guidelines to avoid legal complications. Conversion Rate : Measures the percentage of users who take the desired action, such as filling out a form or scheduling a consultation. For any health issues, always consult a healthcare professional.

Pharma Marketing Network
MARCH 12, 2025
Regulatory Restrictions on Keyword Use Pharmaceutical ads must comply with regulations from organizations like the FDA (U.S.) , MHRA (UK) , and EMA (EU). Using FDA-approved terminology prevents ad disapprovals and regulatory scrutiny. Compliance ensures that keywords align with FDA-approved indications and do not mislead consumers.

Pharma Marketing Network
JANUARY 11, 2025
FDA Guidelines for DTC Ads: The FDA requires ads to present both benefits and risks equally. What are the FDA requirements for direct-to-consumer ads? For any health issues, always consult a healthcare professional. Missteps can lead to hefty fines and reputational damage. Disclaimer: This content is not medical advice.

European Pharmaceutical Review
MARCH 5, 2024
The US Food and Drug Administration (FDA) recently highlighted data integrity issues concerning premarket submissions received for medical devices. Consequently, because “it calls into question the data integrity of the entire file”, FDA asserted that is unable to rely on the data to grant marketing authorisation.

Pharma Marketing Network
APRIL 2, 2025
In 2025, marketing in pharma demands a careful balance between creativity and strict adherence to FDA and international marketing regulations. For any health issues, always consult a healthcare professional. Staying Compliant While Innovating Innovation in pharma marketing cannot come at the cost of regulatory compliance.

pharmaphorum
OCTOBER 4, 2022
On 27th September 2022, the Food and Drug Administration (FDA) issued its final guidance for industry and FDA staff clinical decision support (CDS) software, which has been anticipated since the Center for Devices and Radiological Health (CDRH) listed the guidance as a top priority for fiscal year 2022. Criteria for regulation.

Pharma Marketing Network
MARCH 24, 2025
New FDA guidelines, competitor brand moves, and media spending trends all influence how your brand should show up in-market. These platforms can also monitor FDA press releases, brand safety incidents, and healthcare policy changes in real time. For any health issues, always consult a healthcare professional.

Pharma Marketing Network
MARCH 27, 2025
Brands tiptoed around platforms like Facebook or Twitter, fearful of FDA repercussions, adverse event reporting, and lack of clear guidance. However, the FDA and other regulators have clarified their positions in recent years, particularly around fair balance and risk disclosures. Disclaimer “This content is not medical advice.

Pharma Marketing Network
DECEMBER 31, 2024
Follow FDA guidelines on advertising to ensure your messaging is accurate and ethical. Follow FDA guidelines, ensure accurate messaging, and work closely with legal and regulatory teams to review campaigns. For any health issues, always consult a healthcare professional. How can pharma marketers stay compliant with regulations?
Pharmaceutical Technology
APRIL 11, 2023
Oracle company Cerner Enviza and John Snow Labs have collaborated with the US Food and Drug Administration (FDA) for the development of artificial intelligence (AI) tools for drug safety and real-world evidence studies. Together, Cerner Enviza and John Snow Labs have all the right expertise, data and technology to make it happen.”

Pharma Marketing Network
DECEMBER 4, 2024
Virtual health consultations, mobile apps, and online portals have made it easier to engage patients and healthcare providers alike. Adhering to guidelines from the FDA and other governing bodies ensures that campaigns remain effective without crossing ethical boundaries. Patient-Centric Marketing Patients want to feel heard.

Pharmaceutical Technology
NOVEMBER 7, 2023
Patients who have used the products should consult their healthcare provider and properly discard the product.

Pharma Marketing Network
FEBRUARY 17, 2025
The Value of an Expert Partner: Industry-Specific Expertise: Pharmaceutical marketing companies know the ins and outs of marketing drugs, from branded drug launches to FDA regulations. Can they navigate FDA guidelines, OPDP (Office of Prescription Drug Promotion) regulations, and ensure your campaigns are compliant?

Medical Device Success
MARCH 13, 2011
FDA – 8 – The agency we all complain about so much actually is trying harder to communicate than many pharma and medical device companies. The post Pop Quiz – How many Twitter accounts does the FDA and these Pharma & Medical Device companies have? Had to go to the JNJ site to find it. JNJ should have 8 Twitter feeds.

Pharma Marketing Network
MARCH 6, 2025
Follow FDA and HIPAA Guidelines No misleading claims Every medical statement must be backed by FDA-approved data. Follow FDA, HIPAA, and global regulatory guidelines , and ensure full disclosure of risks and benefits. For any health issues, always consult a healthcare professional. This content is not medical advice.

Nixon Gwilt Law
MAY 29, 2024
The FDA regulates all medical devices, including SaMD and DTx, but recognizes that not all pose the same level of risk to patients. For low-risk software devices, the FDA may exercise enforcement discretion, choosing not to enforce certain regulatory requirements. Which SaMD Device Functions May Qualify for Enforcement Discretion?

Pharma Marketing Network
MARCH 11, 2025
Strict guidelines from the Food and Drug Administration (FDA) and the Federal Trade Commission (FTC) require marketers to ensure transparency and accuracy in all communications. FDA Guidelines and Promotional Review Committees Understanding FDA regulations is critical for all pharma marketing professionals.

Pharmaceutical Technology
NOVEMBER 2, 2022
Ascletis Pharma has submitted an Investigational New Drug (IND) application to the US Food and Drug Administration (FDA) for its drug candidate for Covid-19. The company made the submission after the pre-IND consultation with the regulatory agency. An oral small molecule inhibitor, ASC11 acts on 3-chymotrypsin like protease (3CLpro).

Pharma Marketing Network
NOVEMBER 24, 2024
Always adhere to FDA guidelines and include clear disclaimers in campaigns. Adhering to FDA guidelines, consulting legal experts, and incorporating disclaimers ensure compliance. Use relatable metaphors: Think of content as a bridge connecting pharma companies with patients and HCPs. Non-compliance can cost dearly.

Pharma Marketing Network
MARCH 5, 2025
So how can pharma brands maximize their return on investment (ROI) while ensuring compliance with FDA and HIPAA regulations ? From FDA guidelines to Googles ad policies , failure to comply can result in ad disapprovals, fines, or reputational damage. For any health issues, always consult a healthcare professional.

Legacy MEDSearch
AUGUST 22, 2022
Food and Drug Administration (FDA) has granted 510(k) clearance to the MedWand,” shared MedWand CEO and Co-Founder, Robert Rose. “As With FDA clearance, clinicians and patients everywhere can trust MedWand’s vitals and remote examination tools, and experience telemedicine on an entirely new level.” MedWand Solutions, Inc.

European Pharmaceutical Review
FEBRUARY 22, 2023
1 Consequently, the US Food and Drug Administration (FDA) and other agencies are keen to see “accelerated development” programmes in areas where there is a significant unmet clinical need. He is currently a CMC consultant with an interest in impurities and safety‑based limits. FDA; 2018 [cited 2023Jan]. billion, and rising.

Pharma Marketing Network
MARCH 18, 2025
Follow FDA and Regulatory Guidelines Ensure all PPC ads adhere to FDA, EMA, or MHRA regulations. Following FDA regulations, using fair balance messaging, and targeting verified HCPs helps maintain compliance. For any health issues, always consult a healthcare professional. Disclaimer This content is not medical advice.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content