OSD contract manufacturing market valued at $54.7 billion by 2030
European Pharmaceutical Review
OCTOBER 7, 2022
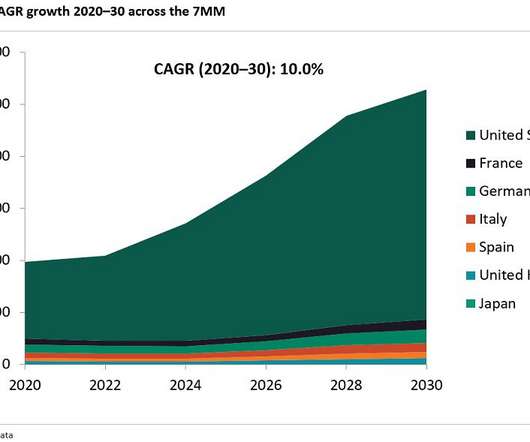
billion by 2030, displaying a compound annual growth rate (CAGR) of 6.0 Slow-release formulations were a popular choice for manufacturers, as these varieties reduce the traditional dosage frequency for patients, who only need to take these medications say, once a week. billion by 2030 appeared first on European Pharmaceutical Review.









































Let's personalize your content