Research predicts 2029 small molecule oncology market leaders
European Pharmaceutical Review
OCTOBER 12, 2023
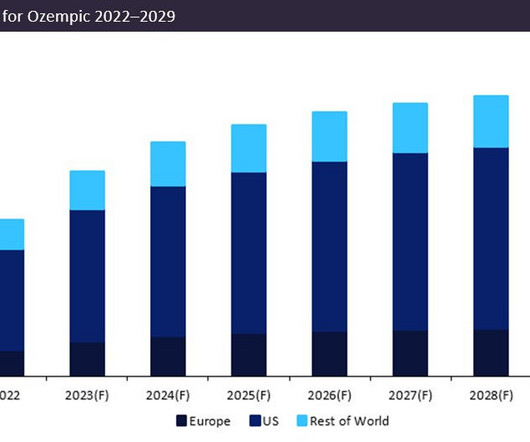
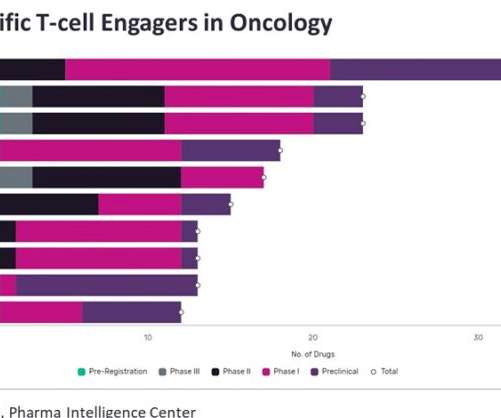
Strong demand for small molecules as treatment for non-small cell lung cancer (NSCLC) in the eight major markets means that the small molecule treatment market for the disease is expected to reach over $15 billion by 2029. billion and a market share of 17 percent by 2029, GlobalData predicted. percent by 2029.















Let's personalize your content