The future of pain medication: are cannabinoids the solution to the opioid epidemic?
Pharmaceutical Technology
JUNE 12, 2023
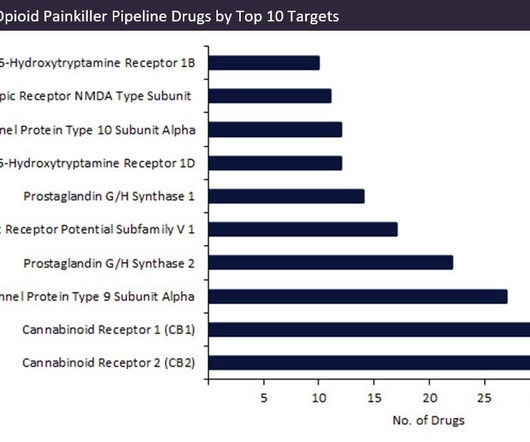
In 2022, the FDA awarded VX-548 breakthrough therapy and fast-track designations for post-operative pain, and the drug is forecast to reach sales of $416m by 2029. A promising example includes Vertex Pharmaceuticals’s VX-548, a sodium channel subunit blocker. Six cannabinoids have recently completed or are undergoing Phase II trials.













Let's personalize your content