NHS announces hospitals will roll out ‘Martha’s Rule’ as part of patient safety initiative
PharmaTimes
MAY 30, 2024
The first phase of the programme will be in place in hospitals across the UK by March 2025
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

PharmaTimes
MAY 30, 2024
The first phase of the programme will be in place in hospitals across the UK by March 2025

pharmaphorum
AUGUST 24, 2022
The biotech has confirmed it will not launch the biosimilar until March 2025 however, honouring the terms of its 2020 agreement with Alexion that resolved litigation over the validity of patents covering Soliris (eculizumab) in the US.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

European Pharmaceutical Review
DECEMBER 19, 2024
For] AI use in life sciences R&D… 2025 will see the industry enter the “plateau of enlightenment” AI use in life sciences R&D has now passed through most major stages of the Gartner Hype Cycle 2 and 2025 will see the industry enter the “plateau of enlightenment.” Internet] wh.gov.

PM360
NOVEMBER 12, 2024
The 2025 Healthcare CMO Summit is an exclusive invitation-only event designed to bring together Chief Medical Officers (CMOs) and innovative suppliers and solution providers. Scheduled for February 3-4, 2025 , at The Ritz-Carlton Dallas, Las Colinas , the summit will address key clinical healthcare challenges and relevant market developments.

PM360
NOVEMBER 12, 2024
The 2025 Healthcare CNO Summit , an exclusive invitation-only event, is scheduled for February 3-4, 2025, at The Ritz-Carlton Dallas, Las Colinas. Quality Safety Controls: Mitigating challenges to ensure patient safety and quality care.

Pharma Marketing Network
OCTOBER 17, 2024
As we approach 2025, the ability of pharmaceutical companies to effectively craft and communicate their brand identity will play a pivotal role in determining their market success. Key Strategies for Pharma Marketing in 2025 As pharmaceutical companies look to 2025, embracing a patient-centric approach will be pivotal.

European Pharmaceutical Review
MARCH 24, 2023
The European Medicines Agency (EMA) has published a report summarising the mid-point achievements of its Regulatory Science Strategy (RSS) to 2025. Regulatory science refers to the scientific disciplines that are applied to the quality, safety and efficacy assessment of medicinal products.

Pharma Marketing Network
OCTOBER 17, 2024
As we approach 2025, the pharmaceutical industry is witnessing a seismic shift in marketing strategies, driven by technological advancements and evolving consumer expectations. As we move towards 2025, regulatory bodies are increasingly focusing on transparency, patient privacy, and ethical marketing practices.

European Pharmaceutical Review
OCTOBER 22, 2024
The agency anticipated that the regulation could become law in Summer 2025. Introduction of this framework was originally announced by the MHRA in January 2023. MHRA shared that it will publish guidance to support implementation of the new regulations prior to the legislation coming into force.

Nixon Gwilt Law
JULY 23, 2024
In the recently released 2025 Medicare Physician Fee Schedule Proposed Rule (the “Proposed Rule”), the Centers for Medicare & Medicaid Services (CMS) created a new reimbursement pathway for “Digital Mental Health Treatment” (DMHT) devices and services. Whether it’s assisting you with drafting comments to the Proposed Rule ( Note!

European Pharmaceutical Review
OCTOBER 6, 2023
Data from Moderna’s mRNA-1083 Phase I/II trial The ongoing Phase I/II clinical trial is evaluating the safety and immunogenicity of mRNA-1083 compared to a standard dose of the influenza vaccine Fluarix, in adults aged 50-64. No new safety concerns were identified for mRNA-1083 compared to the standalone vaccines in the study.

Nixon Gwilt Law
NOVEMBER 8, 2024
CMS issued its 2025 Medicare Physician Fee Schedule (the “2025 MPFS” or “Final Rule”) on November 1, 2024. The Final 2025 MPFS contains some modifications and clarifications to several elements of the original proposal released in July 2024 (the “Proposed Rule”), which we discussed in this earlier post.

Nixon Gwilt Law
JULY 23, 2024
In the recently released 2025 Medicare Physician Fee Schedule Proposed Rule (the “Proposed Rule”), the Centers for Medicare & Medicaid Services (CMS) created a new reimbursement pathway for “Digital Mental Health Treatment” (DMHT) devices and services. Whether it’s assisting you with drafting comments to the Proposed Rule ( Note!

European Pharmaceutical Review
NOVEMBER 2, 2023
The FDA said its approval of Wezlana is based on “a comprehensive review of scientific evidence demonstrating it is highly similar to Stelara and that there are no clinically meaningful differences between the two products in terms of safety, purity and potency.” Like Stelara, the most serious known side effect of Wezlana is infection.

European Pharmaceutical Review
AUGUST 30, 2023
The following new measures are set to be introduced on 1 January 2025. Implementing compliant medicine packaging and labelling With the new measures set to come into force at the start of 2025, joint EU/UK packs can no longer enter the supply chain.

European Pharmaceutical Review
APRIL 1, 2024
REGENXBIO’s Phase I/IIa trial investigated the safety and tolerability of a single dose of subretinal ABBV-RGX-314 for wet age-related macular degeneration (wet AMD). Regulatory submissions are expected in late 2025 through the first half of 2026, REGENXBIO confirmed.
European Pharmaceutical Review
JUNE 13, 2024
Potential of Mim8 The safety profile of Mim8 was in line with previous trials. Data from the Phase III FRONTIER programme will be shared in future conferences and publications in 2024 and 2025, Novo Nordisk added. No deaths or thromboembolic events were observed, the study found.

European Pharmaceutical Review
SEPTEMBER 14, 2023
Once operational in early 2025, the partnership will reduce emissions by an estimated 20,000 tonnes CO 2 equivalent (CO 2 e). The pharma company’s sites in Macclesfield, Cambridge, Luton and Speke will be supplied by energy from the biomethane facility.

pharmaphorum
SEPTEMBER 9, 2022
Prior to the launch of those drugs, pharmacological therapy for obesity was something of a backwater, constrained by tolerability and safety issues for older, generic drugs, and lacklustre take-up of newer therapies with limited efficacy. billion in 2025. Novo Nordisk recently predicted its obesity sales could reach $3.7

European Pharmaceutical Review
OCTOBER 17, 2024
Thus, ARS Pharmaceuticals anticipates approval by Q1 2025 in the UK and launch by summer 2025. neffy, Epinephrine Nasal Spray, Demonstrates a Positive Efficacy and Safety Profile for the Treatment of Allergic Reactions in Pediatric Patients at Risk of Anaphylaxis: Phase 3 Study Results. Motohiro E, Kento T, Kyohei T , et al.
pharmaphorum
SEPTEMBER 21, 2022
Approval of the first-in-class TSLP inhibitor wasn’t considered a shoo-in, as Tezspire failed one of its two pivotal phase 3 trials, but the Commission and FDA have concluded that the totality of the data – which also included a positive phase 2b study – was enough to show efficacy and safety. billion or more.

European Pharmaceutical Review
JUNE 12, 2024
The main objective was to evaluate the efficacy and safety of MBK-01 compared to fidaxomicin, providing significant insights into the potential of MBK-01 as a treatment for primary or recurrent CDI and contributing to the advancement of microbiome-based therapies. The study included 92 adult patients with confirmed CDI.
European Pharmaceutical Review
FEBRUARY 17, 2023
The company believes this will be sufficient to fund its planned operations and expenses into the first quarter of 2025. Dr Sartor noted that there is increasing evidence that targeted alpha therapies for prostate cancer is effective even for “patients who progress on or after lutetium-based PSMA therapies.”
European Pharmaceutical Review
NOVEMBER 4, 2024
The chapter covers endotoxin testing using non-animal derived reagents and is set to become official in May 2025, the organisation announced. Chapter <86>] is set to become official in May 2025″ USP confirmed plans to remove the rabbit pyrogen test (RPT) from its monographs in June this year, during the Ph.

pharmaphorum
DECEMBER 14, 2022
Tentative approval means Lumryz has satisfied all the FDA requirements for efficacy, safety, and quality standards, but it can’t be fully approved until a patent or other exclusivity expires. Two decades of exclusivity. The best-case scenario? Jazz and Avadel settle in court soon, giving people with narcolepsy another option for treatment.
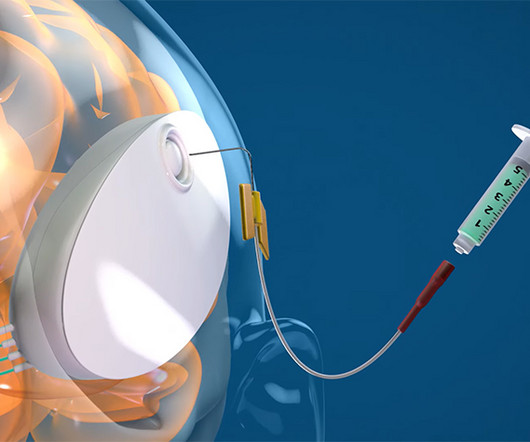
Medgadget
SEPTEMBER 29, 2023
We aim to optimize the device’s design for enhanced safety, reliability, and patient comfort and are targeting FDA IND submission and approval in late 2024 to conduct a Phase I first-in-human study in 2025. The NeuroPASS device represents a significant leap forward in addressing the challenges posed by the blood-brain barrier.

Copyright Clearance Center
DECEMBER 10, 2024
He highlighted how collaborations on harmonizing data standards have improved regulatory review practices and enabled predictive models to enhance drug safety. The Pistoia Alliance is now welcoming registrations for its next member conference in London, 25-26 March 2025.
European Pharmaceutical Review
MARCH 14, 2024
The safety profile in the open-label extension was consistent with the randomised period and with the overall population of patients with IgAN. Topline data from Vera’s ORIGIN Phase III trial of atacicept is expected in the first half of 2025. 3 About the interviewee Dr Marshall Fordyce is CEO of Vera Therapeutics. cited 2024Mar].
European Pharmaceutical Review
MARCH 14, 2024
The safety profile in the open-label extension was consistent with the randomised period and with the overall population of patients with IgAN. Topline data from Vera’s ORIGIN Phase III trial of atacicept is expected in the first half of 2025. 3 About the interviewee Dr Marshall Fordyce is CEO of Vera Therapeutics. cited 2024Mar].

pharmaphorum
SEPTEMBER 14, 2022
According to the Paris-based biotech, SPVN06 should start trials in RP towards the end of this year, with safety data due in 2023 and efficacy proof-of-concept in 2025. The current schedule is for trials to start in 2024, with first safety and efficacy data coming a year later.

Referral MD
MAY 19, 2023
Blockchain will improve healthcare safety and transparency. Innovation and Security Implications of Blockchain in Health Care Innovation and Safety Blockchain’s impact on innovation and security depends on what it adds to a non-blockchain environment. Healthcare blockchain technology safety research should also be prioritized.

Pharmaceutical Technology
JANUARY 8, 2023
The Swiss pharma giant is running a Phase III trial (NCT05595642) with its anti-IL33 antibody astegolimab for patients with COPD, with an anticipated completion date in 2025, but a Phase II trial is expected to complete in 2024 (NCT05037929). Ongoing efforts. Have biologics for COPD arrived? Not yet, but soon, industry players hope.
pharmaphorum
SEPTEMBER 20, 2022
The phase 1/3 ROSALIA study showed that the copycat denosumab matched Amgen’s brand on multiple pharmacokinetics, pharmacodynamics, efficacy, safety, and immunogenicity measures in postmenopausal women with osteoporosis, said Sandoz in a statement.

pharmaphorum
NOVEMBER 25, 2022
The Parma site, as yet only nine months into construction, is due to become operational from 2024, with FDA, IFA, and EMA approvals due to be sought for 2025. Going beyond the standards of care; improving drug safety and efficacy. But to make people safe requires a lot of research.

Pharmaceutical Technology
MAY 25, 2023
According to the press release, the MHRA will be the first drug safety regulator in the world to pilot such a biobank. The first patients will have their genetic makeup sequenced in Spring 2024 and this data will be shared in 2025. While the pilot program will begin on 1 June, participant recruitment will start on 1 September.

Contrarian Sales Techniques
FEBRUARY 24, 2023
RM1 billion will be allocated to strengthen domestic safety, including acquiring more than 2,100 units of body cameras for the Royal Malaysia Police. The Green Technology Financing Scheme (GTFS) is improved, with the guaranteed value increased to RM3 billion until 2025.

Scott Burrows
DECEMBER 13, 2024
Heres to embracing every opportunity, conquering every challenge, and making 2025 a year to remember. With a clear vision, a positive mindset, and unwavering grit, youre setting the stage for a year filled with growth, resilience, and extraordinary achievement. Dream big, work hard, and step boldly into the possibilities aheadyouve got this!
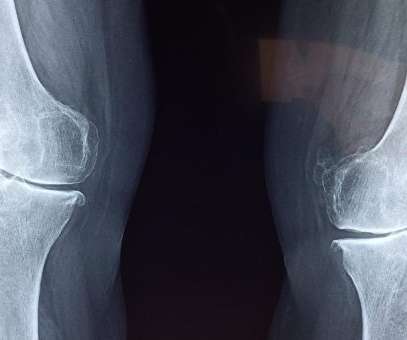
pharmaphorum
AUGUST 19, 2022
If all goes well, the aim is to bring resiniferatoxin to market in 2025. It will recruit people with osteoarthritis who have exhausted other treatment options but are still experiencing moderate to severe pain. Along with knee osteoarthritis, Grünenthal also plans to test resiniferatoxin in other joints affected by the condition.

European Pharmaceutical Review
FEBRUARY 7, 2023
From 31 January 2025, new and ongoing clinical trials in the EU must be conducted in compliance with the CTR. In addition, the devices must not present an unacceptable risk to patient and user health and safety as established in Articles 94 and 95 of the MDR. Authorisation of the clinical trial, may, however, be obtained later.
pharmaphorum
NOVEMBER 9, 2022
The keynote address of the event entire came from Stella Kyriakides, commissioner for health and food safety at the European Commission. In order to do this, an EU network is in the process of being established between national comprehensive cancer centres and anticipated to be properly in place by 2025. Her opener?

pharmaphorum
JANUARY 4, 2023
The EC is hoping that, by the end of its current mandate on 31 October 2024, the legislative process will be completed, with the EHDS becoming operational in 2025. providing personalised healthcare consisting in assessing, maintaining, or restoring the state of health of individuals. When will it come into force?

Clarivate
MAY 13, 2024
After staying relatively flat for several years, enrollment growth took off in 2021 after an expansion in federal subsidies for silver-level plans passed in the American Rescue Plan and was then renewed through 2025 in the Inflation Reduction Act.

European Pharmaceutical Review
JUNE 27, 2024
Dr Feller highlighted that Geron anticipates marketing authorisation application (MAA) review “to be completed in early 2025 and look forward to the potential of bringing [the treatment for transfusion-dependent anaemia in LR-MDS] to patients in Europe”.
pharmaphorum
SEPTEMBER 11, 2022
Analysts at Mizuho have suggested that competition from BMS’ drug could trim Otezla’s 2025 sales from $3 billion to $2 billion, although Amgen is attempting to defend its product by expanded the label of the drug to include mild psoriasis. Otezla was acquired by Amgen for $13.4
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content