NHS announces hospitals will roll out ‘Martha’s Rule’ as part of patient safety initiative
PharmaTimes
MAY 30, 2024
The first phase of the programme will be in place in hospitals across the UK by March 2025
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

PharmaTimes
MAY 30, 2024
The first phase of the programme will be in place in hospitals across the UK by March 2025

pharmaphorum
AUGUST 24, 2022
The biotech has confirmed it will not launch the biosimilar until March 2025 however, honouring the terms of its 2020 agreement with Alexion that resolved litigation over the validity of patents covering Soliris (eculizumab) in the US.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

European Pharmaceutical Review
DECEMBER 19, 2024
1 These concerns are held alongside the promise of this technology and its potential for improving efficiency, enhancing patient outcomes, and addressing critical challenges like staffing shortages. In 2025, organisations must focus on addressing data integrity challenges. Internet] wh.gov. Available from: [link] 7. 2024; 177: 108632.

PM360
NOVEMBER 12, 2024
The 2025 Healthcare CNO Summit , an exclusive invitation-only event, is scheduled for February 3-4, 2025, at The Ritz-Carlton Dallas, Las Colinas. Quality Safety Controls: Mitigating challenges to ensure patient safety and quality care.

Pharma Marketing Network
OCTOBER 17, 2024
As we approach 2025, the ability of pharmaceutical companies to effectively craft and communicate their brand identity will play a pivotal role in determining their market success. Key Strategies for Pharma Marketing in 2025 As pharmaceutical companies look to 2025, embracing a patient-centric approach will be pivotal.

European Pharmaceutical Review
OCTOBER 22, 2024
The UK is set to be the first country to introduce a new regulatory framework for innovative products manufactured at or close to the point of patient care, says the Medicines and Healthcare products Regulatory Agency (MHRA). The agency anticipated that the regulation could become law in Summer 2025.

pharmaphorum
SEPTEMBER 9, 2022
It also marks the first move by Kry beyond the primary care category, where it provides virtual GP consultation services to millions of patients across various European countries, into tools for the treatment and prevention of chronic diseases. billion in 2025. Novo Nordisk recently predicted its obesity sales could reach $3.7

Pharma Marketing Network
OCTOBER 17, 2024
As we approach 2025, the pharmaceutical industry is witnessing a seismic shift in marketing strategies, driven by technological advancements and evolving consumer expectations. As patients become more digitally savvy, pharma companies are investing in creating seamless digital experiences that cater to their needs and preferences.

European Pharmaceutical Review
APRIL 1, 2024
REGENXBIO’s Phase I/IIa trial investigated the safety and tolerability of a single dose of subretinal ABBV-RGX-314 for wet age-related macular degeneration (wet AMD). The two-year data showed that ABBV-RGX-314 provided stable or improved visual acuity and retinal thickness in patients.

European Pharmaceutical Review
NOVEMBER 2, 2023
The FDA said its approval of Wezlana is based on “a comprehensive review of scientific evidence demonstrating it is highly similar to Stelara and that there are no clinically meaningful differences between the two products in terms of safety, purity and potency.” Like Stelara, the most serious known side effect of Wezlana is infection.
European Pharmaceutical Review
JUNE 13, 2024
Significant reductions of treated bleeding episodes in haemophilia A In the FRONTIER 2 trial, treatment once-weekly and once-monthly with Mim8 provided a 97 percent and 99 percent reduction in treated bleeds in patients with no prior prophylaxis treatment, respectively. This was compared to prior coagulation factor prophylaxis.

European Pharmaceutical Review
OCTOBER 6, 2023
For example: higher patient compliance, easier administration and greater convenience. Data from Moderna’s mRNA-1083 Phase I/II trial The ongoing Phase I/II clinical trial is evaluating the safety and immunogenicity of mRNA-1083 compared to a standard dose of the influenza vaccine Fluarix, in adults aged 50-64.

Nixon Gwilt Law
JULY 23, 2024
In the recently released 2025 Medicare Physician Fee Schedule Proposed Rule (the “Proposed Rule”), the Centers for Medicare & Medicaid Services (CMS) created a new reimbursement pathway for “Digital Mental Health Treatment” (DMHT) devices and services. for conditions other than substance abuse disorder, insomnia, and depression)?
pharmaphorum
SEPTEMBER 21, 2022
The European Commission has followed the lead of the US FDA and approved AstraZeneca’s Tezspire as an add-on maintenance therapy for patients with severe asthma, becoming the first and only biologic that can be used in all patients, and not restricted to those with specific forms of the disease. billion or more.

Nixon Gwilt Law
NOVEMBER 8, 2024
CMS issued its 2025 Medicare Physician Fee Schedule (the “2025 MPFS” or “Final Rule”) on November 1, 2024. The Final 2025 MPFS contains some modifications and clarifications to several elements of the original proposal released in July 2024 (the “Proposed Rule”), which we discussed in this earlier post.

Nixon Gwilt Law
JULY 23, 2024
In the recently released 2025 Medicare Physician Fee Schedule Proposed Rule (the “Proposed Rule”), the Centers for Medicare & Medicaid Services (CMS) created a new reimbursement pathway for “Digital Mental Health Treatment” (DMHT) devices and services. for conditions other than substance abuse disorder, insomnia, and depression)?

European Pharmaceutical Review
OCTOBER 17, 2024
These factors may increase the likelihood that patients and caregivers carry it regularly. Thus, ARS Pharmaceuticals anticipates approval by Q1 2025 in the UK and launch by summer 2025. What is significant about approval of EURneffy as the first new delivery method in over three decades for adrenaline?

European Pharmaceutical Review
JUNE 12, 2024
The study included 92 adult patients with confirmed CDI. The main objective was to evaluate the efficacy and safety of MBK-01 compared to fidaxomicin, providing significant insights into the potential of MBK-01 as a treatment for primary or recurrent CDI and contributing to the advancement of microbiome-based therapies.
European Pharmaceutical Review
FEBRUARY 17, 2023
Dr Sartor noted that there is increasing evidence that targeted alpha therapies for prostate cancer is effective even for “patients who progress on or after lutetium-based PSMA therapies.” The Phase II trial is expected to evaluate approximately 100 patients with four treatment cycles per patient occurring every eight weeks.

pharmaphorum
DECEMBER 14, 2022
When patients experience intense emotion, either positive or negative, they are rendered unconscious. In order to bring it to market, a special program called Risk Evaluation and Mitigation Strategy (REMS) had to be put in place, where only eligible patients could access the drug through a very strict process. One more hurdle to clear.

Pharmaceutical Technology
JANUARY 8, 2023
While options like bronchodilator inhalers are available for COPD patients, these have limitations. Biologics have the potential to impact disease-driving processes if we hit the right target in the right patient,” Adcock continues. Still, patients require an individual-led approach. Biologics: From asthma to COPD?

Referral MD
MAY 19, 2023
Blockchain may help organizations securely share healthcare data while protecting patient privacy. The health system is also moving towards a patient-centered approach prioritizing accessible services and adequate healthcare resources. Blockchain improves healthcare organizations’ patient care and facilities.
Medgadget
SEPTEMBER 29, 2023
This layer of specialized endothelium significantly restricts which drug molecules can enter the brain, normally greatly limiting treatment options for patients with brain-based disease. In addition, it isn’t sustainable to have patients in-hospital for months at a time seeking this type of treatment.
European Pharmaceutical Review
MARCH 14, 2024
This month, new data from Novo Nordisk has revealed that semaglutide could become the first GLP-1 treatment option for patients with type 2 diabetes and chronic kidney disease (CKD). Up to fifty percent of IgAN patients progress to end-stage renal disease, requiring dialysis or transplant.
European Pharmaceutical Review
MARCH 14, 2024
This month, new data from Novo Nordisk has revealed that semaglutide could become the first GLP-1 treatment option for patients with type 2 diabetes and chronic kidney disease (CKD). Up to fifty percent of IgAN patients progress to end-stage renal disease, requiring dialysis or transplant.

Copyright Clearance Center
DECEMBER 10, 2024
The survey was conducted at the Alliances annual US conference held this year in Philadelphia, which gathered more than 200 industry experts to collaborate on topics such as AI regulation, harmonization of data standards, and patient-centricity. The best way to overcome any lingering adoption hurdles is together.

pharmaphorum
SEPTEMBER 14, 2022
SparingVision’s lead candidates are both targeting inherited retinal diseases (IRDs), a group which already has a gene therapy on the market in Novartis’ Luxturna (voretigene neparvovec), which was approved by the FDA in 2017 for biallelic RPE65 mutation-associated retinal dystrophy, a group which includes a small group of RP patients.

PM360
AUGUST 9, 2022
How can we help ensure patients continue to take their medications as prescribed? The reasons why a patient may not take their medication are plentiful, and as a result so are the solutions now available to attempt to solve this issue. billion in 2025. But it is not a simple answer. from 2021 and reach $4.2
pharmaphorum
NOVEMBER 9, 2022
The keynote address of the event entire came from Stella Kyriakides, commissioner for health and food safety at the European Commission. It is, she said, the uniting of industry with researchers and researchers with patients, and so forth, that is at the heart of this journey currently being undertaken. Her opener?

Pharmaceutical Technology
MAY 25, 2023
The Medicines and Healthcare products Regulatory Agency (MHRA) aims to launch a pilot genetic biobank that will gather patient data to associate drug-related adverse events to their genetic makeup. According to the press release, the MHRA will be the first drug safety regulator in the world to pilot such a biobank.

pharmaphorum
NOVEMBER 25, 2022
The Parma site, as yet only nine months into construction, is due to become operational from 2024, with FDA, IFA, and EMA approvals due to be sought for 2025. Going beyond the standards of care; improving drug safety and efficacy. But to make people safe requires a lot of research.
pharmaphorum
AUGUST 19, 2022
Grünenthal has moved its painkiller resiniferatoxin into phase 3 trials in patients with osteoarthritis, hoping to find an option that sidesteps the side effects and addictive potential of opioid drugs. If all goes well, the aim is to bring resiniferatoxin to market in 2025.

European Pharmaceutical Review
JUNE 27, 2024
Immunotherapy in oncology “The addition of pembrolizumab to chemotherapy represents a new frontline therapeutic option for patients with primary advanced or recurrent endometrial carcinoma” Moreover, last week saw US regulatory approval of a combination treatment for endometrial cancer : KEYTRUDA (pembrolizumab) plus chemotherapy.

pharmaphorum
JANUARY 4, 2023
The intention is to streamline the process for patients wishing to access their medical records via electronic health record systems (“EHR systems”) and to facilitate the ability for researchers, innovators, and policy makers to use the data in a trusted and secure way that preserves privacy. When will it come into force?
pharmaphorum
SEPTEMBER 20, 2022
The phase 1/3 ROSALIA study showed that the copycat denosumab matched Amgen’s brand on multiple pharmacokinetics, pharmacodynamics, efficacy, safety, and immunogenicity measures in postmenopausal women with osteoporosis, said Sandoz in a statement.
pharmaphorum
SEPTEMBER 11, 2022
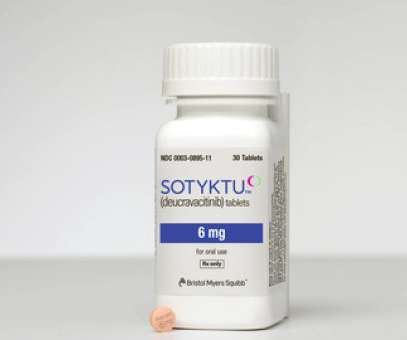
Results from the POETYK PSO-1 and POETYK PSO-2 showed that 59% and 54% respectively of patients treated with Sotyktu achieved 75% skin clearance, versus 35% and 40% for Otezla. “We believe Sotyktu is a breakthrough in the treatment of patients with this condition, and we’re excited about its potential in other immune-mediated diseases.”

European Pharmaceutical Review
FEBRUARY 7, 2023
From 31 January 2025, new and ongoing clinical trials in the EU must be conducted in compliance with the CTR. In addition, the devices must not present an unacceptable risk to patient and user health and safety as established in Articles 94 and 95 of the MDR. Authorisation of the clinical trial, may, however, be obtained later.

Pharmaceutical Technology
JUNE 1, 2023
In addition, the FDA previously rejected Intercept’s OCA NDA, with the federal agency issuing a complete response letter (CRL) requesting longer-term post-interim analysis efficacy and safety data from the ongoing pivotal Phase III REGENERATE trial (NCT02548351) in June 2020. risk reduction compared with placebo).

Contrarian Sales Techniques
NOVEMBER 18, 2024
In Budget 2025, the Ministry of Health received an allocation of RM45.3 These subsidies enable clinics to procure medications in bulk at negotiated prices, allowing patients to pay as little as RM1 or RM5 for a full course of treatment. It’s a safety net that quietly supports the health of the nation. billion, marking a 9.8%

Salesforce
JUNE 28, 2024
These include: Data governance Data analytics Data collection Patient safety Regulatory compliance When done correctly, these tasks set standards for how data is collected, stored, used, and shared. This is valuable, especially when you consider that recruiting patients can be one of the biggest challenges. Back to top.

Clarivate
OCTOBER 10, 2022
Clarivate oncology experts share key takeaways from the 2022 ESMO Congress and their expected impact on the drug treatment landscape and patients. Positive outcomes in a key patient segment could broaden TRODELVY’s uptake in metastatic breast cancer. Market impact.
Contrarian Sales Techniques
FEBRUARY 25, 2023
The film documents the efforts of activists, doctors, and patients to overcome these barriers and increase access to affordable treatment. The pharmaceutical industry in India is growing rapidly and is expected to reach a value of US$100 billion by 2025, with a significant portion of that growth driven by domestic demand.

Medico Reach
AUGUST 30, 2022
New York Presbyterian Hospital’s ‘Patient Stories’. Healthcare advertisements that feature patient experiences can evoke powerful emotions. It hosted a campaign that highlighted the real-life experiences of patients. The highlighted blog featured personal accounts from patients, their families, and Mayo Clinic employees.

Clarify Health
JULY 13, 2020
The panel included Bhash Parasuraman, PhD, Vice President and BU Lead, Rare Disease Patient & Health Impact, Pfizer , Calum MacRae, MD, PhD, Professor of Medicine at Harvard Medical School , and Surya Singh, MD, President, Singh Healthcare Advisors. By 2025, several new gene and cell therapies will be coming to market.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content