Despite benefits, long-acting injectables remain underutilised for schizophrenia
Pharmaceutical Technology
JULY 28, 2022
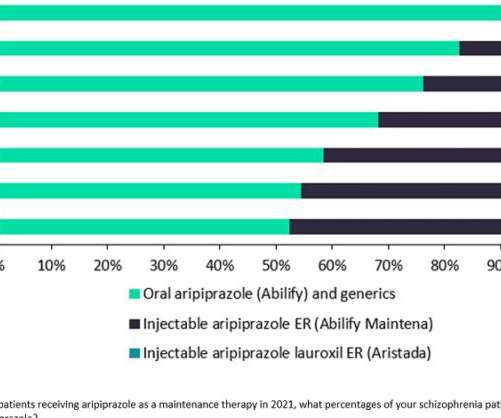
Prescription of daily oral aripiprazole continues to dominate the use of Otsuka and Lundbeck’s once-monthly LAI formulation Abilify Maintena and Alkermes’ Aristada, which can be administered every six weeks. GlobalData forecasts that it will launch in the US and 5EU next year.













Let's personalize your content