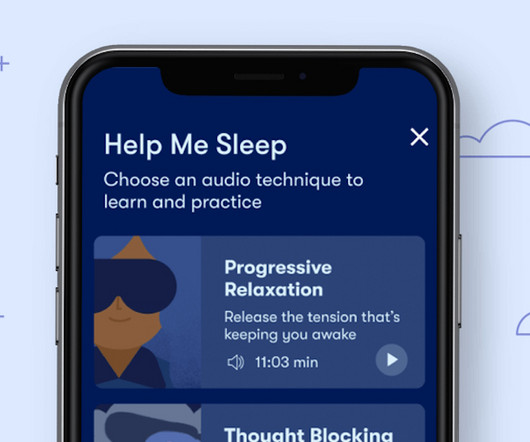
US insomniacs have a new prescription DTx option
pharmaphorum
AUGUST 22, 2024
US insomniacs have a new prescription DTx option Phil.Taylor Thu, 22/08/2024 - 07:59 Bookmark this
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
pharmaphorum
AUGUST 22, 2024
US insomniacs have a new prescription DTx option Phil.Taylor Thu, 22/08/2024 - 07:59 Bookmark this

PharmaTimes
FEBRUARY 1, 2024
Prescriptions ordered via the app are expected to save around 1.85 million hours in 2024
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

MedCity News
FEBRUARY 4, 2024
2024 stands at a critical juncture in the battle against soaring pharmacy costs. In 2023, prescription drug costs rose by 8.4%, a 31% increase from the prior year. By enforcing transparency, PBMs would need to release detailed information on prescription drug spending. Transparency and reporting requirements.

MedCity News
OCTOBER 11, 2024
For 2025, the average star rating for Medicare Advantage Prescription Drug contracts was 3.92, compared to 4.07 for 2024, 4.14 for 2023 and 4.37 The post Medicare Advantage Star Ratings Drop Again appeared first on MedCity News.

MedCity News
JULY 12, 2023
CVS Caremark and GoodRx created a new program called Caremark Cost Saver, which will become available starting January 1, 2024. Through the program, eligible CVS Caremark commercially insured members will have access to GoodRx’s prescription pricing on generic medications.

MedCity News
AUGUST 1, 2023
in 2024, down from $56.49 The average total monthly premium for Medicare Part D coverage is expected to be $55.50 in 2023, according to CMS.

MedCity News
AUGUST 8, 2023
Through Scripta Insights’ app, members of its customers can input the medications they’re taking, and the company will provide a savings report that will show different options on how they can save on their prescriptions. Starting in 2024, Cost Plus Drugs may show up as an option in the savings report.

PM360
DECEMBER 20, 2024
Swoop Launched in September 2024, the Swoop HCP Pro Suite is revolutionizing how pharmaceutical marketers engage with healthcare providers (HCPs). By tailoring messaging frequency and content to align with patient care journeys, marketers eliminate waste, improve engagement, and drive higher prescription lift.

Healthcare Success
JANUARY 2, 2024
Now that the holidays have drawn to a close, it’s time to return to your 2024 marketing plan and make adjustments that ensure your business is taking advantage of the latest trends in healthcare marketing to reach patients and HCPs more effectively and make a lasting impression.

Pharmacy Times
MARCH 5, 2024
The continuous glucose monitoring system will be available for purchase online and without a prescription starting in summer 2024.

PM360
DECEMBER 20, 2024
Pharmacies are also feeling the impact, as unaffordable medication costs lead to prescription abandonment, undermining long-term care plans. The State of Patient Access and Affordability A 2024 study 1 found that patients and providers both consider quick and accurate insurance verification their top priority.

Pharmaceutical Technology
SEPTEMBER 3, 2024
New data provides more evidence on choosing the ideal approach for prescription of beta blockers for myocardial infarction.

PM360
MAY 23, 2024
For example, Bryan’s leadership played a pivotal role in the design and development of Veeva Compass’ modern data products—Veeva Compass Prescriber and National—featuring prescriber -and national- level projections based on both prescription and medical claims. As rare diseases affect about 1 in 10 people in the U.S.,

PM360
FEBRUARY 8, 2024
Saatchi & Saatchi Wellness and TOM (Tikkun Olam Makers) The Prescription Paper Pill Bottle An innovative alternative to plastic, the Prescription Paper Pill Bottle is 100% compostable with no artificial glue and no toxic dye. It’s open-source design meets FDA requirements for safety and protection.

PM360
NOVEMBER 13, 2023
When it comes to platforms used by HCPs to better understand prescription drug pricing information, GoodRx is second only to a provider’s electronic health record (EHR). Is GoodRx in Your 2024 HCP Marketing Plan? Click here to learn more about GoodRx HCP media solutions and give us a try in your 2024 HCP marketing plan.

Pharmaceutical Commerce
JANUARY 24, 2024
The FDA assigned Autolus Therapeutics' biologics license application for obecabtagene autoleucel with a Prescription Drug User Fee Act date of November 16, 2024.
Pharmaceutical Commerce
NOVEMBER 4, 2024
With a 26% decline in prescription drug plans from 2024 to 2025, navigating potential gaps and accessing care remains vital for low income patients.

PharmExec
FEBRUARY 21, 2024
The FDA assigned the biologics license application for linvoseltamab to treat relapsed/refractory multiple myeloma with a Prescription Drug User Fee Act of August 22, 2024.

Pharmaceutical Commerce
FEBRUARY 20, 2024
The FDA has assigned Servier’s New Drug Application for vorasidenib to treat IDH-mutant gliomas with a Prescription Drug User Fee Act action date of August 20, 2024.

Pharmaceutical Commerce
NOVEMBER 10, 2023
Blue Fin Group exec speaks on his upcoming feature.

Pharmacy Times
FEBRUARY 7, 2024
The FDA assigned a Prescription Drug User Fee Act (PDUFA) goal date of October 8, 2024.

PharmExec
FEBRUARY 20, 2024
The FDA assigned the supplemental new drug application for Krazati (adagrasib) plus cetuximab in patients with locally advanced or metastatic colorectal cancer with a Prescription Drug User Fee Act goal date of June 21, 2024.

PharmExec
FEBRUARY 6, 2024
The FDA assigned a Prescription Drug User Fee Act action date of June 7, 2024, to an application that would expand the indication of Arexvy to include adults 50-59 years with an increased risk of respiratory syncytial virus-related lower respiratory tract disease.

Nixon Gwilt Law
JANUARY 16, 2024
What can digital health innovators and investors anticipate for the industry in 2024? What can digital health innovators and investors anticipate for the industry in 2024? Read on to find out what else is on our Legal Vision Board for 2024! How is 2024 Shaping up for Your Business?
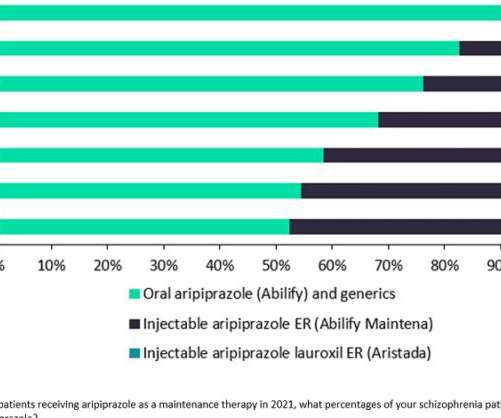
Pharmaceutical Technology
JULY 28, 2022
Prescription of daily oral aripiprazole continues to dominate the use of Otsuka and Lundbeck’s once-monthly LAI formulation Abilify Maintena and Alkermes’ Aristada, which can be administered every six weeks. GlobalData forecasts that it will launch in the US and 5EU next year.

European Pharmaceutical Review
OCTOBER 17, 2024
In August 2024, the European Commission approved EURneffy (adrenaline nasal spray) in the EU as the first needle-free emergency option to treat anaphylaxis. Poster Presented at the 2024 AAAAI Annual meeting, DC, US. This decision was granted a couple of weeks following approval by the US and Drug Administration (FDA).

Pharmaceutical Commerce
DECEMBER 19, 2023
Merck’s Biologics License Application for V116, a 21-valent pneumococcal conjugate vaccine, was given a Prescription Drug User Fee Act (PDUFA) date of June 17, 2024.

Nixon Gwilt Law
JANUARY 16, 2024
What can digital health innovators and investors anticipate for the industry in 2024? Read on to find out what else is on our Legal Vision Board for 2024! Rebecca says, “ New and existing companies will offer GLP-1 prescriptions for weight loss. How is 2024 Shaping up for Your Business?

PharmExec
DECEMBER 19, 2023
Merck’s Biologics License Application for V116, a novel 21-valent pneumococcal conjugate vaccine has been given a Prescription Drug User Fee Act (PDUFA) date of June 17, 2024.

PharmExec
NOVEMBER 30, 2023
Supplemental new drug application for roflumilast cream 0.15% to treat atopic dermatitis in patients 6 years of age and older was assigned a Prescription Drug User Fee Act target action date of July 07, 2024.

PM360
SEPTEMBER 21, 2023
As many in the industry now know, the Inflation Reduction Act (IRA) is the most significant reform of Medicare prescription drug coverage since the creation of Part D and has wide-ranging implications for the healthcare industry. CMS will present an initial offer for each drug’s maximum fair price (MFP) by February 1, 2024.
PM360
OCTOBER 22, 2024
Improving our collective understanding of the patient’s overall healthcare experience is necessary to effectively deliver prescription support. How can we ensure that vital prescription information reaches patients when they need it? 4 These statistics tell a story of trust, concern, and changing dynamics in healthcare communication.

Nixon Gwilt Law
JUNE 24, 2024
The Digital Therapeutics Alliance Summit 2024 , held in Washington, D.C., Instead of limiting themselves under a prescription-based model, founders should explore alternative revenue streams. Collaborative ventures with school systems, employers, payers, and health systems are good opportunities to consider.

Nixon Gwilt Law
JUNE 24, 2024
The Digital Therapeutics Alliance Summit 2024 , held in Washington, D.C., Instead of limiting themselves under a prescription-based model, founders should explore alternative revenue streams. Collaborative ventures with school systems, employers, payers, and health systems are good opportunities to consider.

PharmExec
FEBRUARY 20, 2024
The FDA set a Prescription Drug User Fee Act date during Q4 of 2024 for the biologics license application for datopotamab deruxtecan in patients with previously treated advanced nonsquamous non-small cell lung cancer.

pharmaphorum
NOVEMBER 2, 2022
Around 70% of new prescriptions for the drug are for patients who haven’t received this type of therapy in the past, with fewer than 10% switching from Trulicity, said Lilly’s chief financial officer Anat Ashkenazi on the call.

European Pharmaceutical Review
JULY 4, 2024
1 For use as prescription drugs, a botanical product must be approved by the FDA: to date, only two have gained this approval. The MA pathway is applicable for herbal medicines designed to treat more serious health conditions and require a medical prescription or the supervision of a medical practitioner. 2024 [cited 2024May].

Pharmaceutical Technology
MAY 19, 2023
The prescription drug user fee act (PDUFA) date is expected to be set as January 2024. The new investigational therapeutic product candidate STS101 is a nasal powder formulation of dihydroergotamine mesylate (DHE), an anti-migraine drug, which is given through the company’s nasal delivery device.

PM360
NOVEMBER 21, 2024
In Korlym’s 12th year on the market, the team was able to maximize the revised positioning, new campaigns, and new educational initiatives to increase new prescription demand by almost 50% and revenue by 39% (Q2 2024 vs. Q2 2023).*

PM360
MAY 21, 2024
Phreesia research shows that 25% of patients do not have a good understanding of the next steps around their prescription medications, and 29% aren’t sure how to manage their medical condition immediately following a healthcare visit.

Pharmaceutical Technology
JANUARY 6, 2023
The regulatory body has assigned a Prescription Drug User Fee Act (PDUFA) date for its decision in the third quarter of this year. If approved, nirsevimab will become the first single-dose preventative against RSV lower respiratory tract disease for the 2023/2024 RSV season in the US.

Nixon Gwilt Law
JUNE 27, 2024
By name alone, “Food as Medicine” can be misunderstood as a treatment replacement for other medications or prescriptions. But the broader perception of FaM isn’t completely free from scrutiny by medical and public communities yet. Others have taken to calling it “Food as Health,” “Nutritional Therapy,” etc. to avoid confusion.

Nixon Gwilt Law
JUNE 27, 2024
By name alone, “Food as Medicine” can be misunderstood as a treatment replacement for other medications or prescriptions. But the broader perception of FaM isn’t completely free from scrutiny by medical and public communities yet. Others have taken to calling it “Food as Health,” “Nutritional Therapy,” etc. to avoid confusion.
Pharmaceutical Technology
JUNE 15, 2023
The regulator has set 13 February 2024 as a Prescription Drug User Fee Act goal date for the review of Ipsen’s sNDA. The Onivyde regimen comprises Onivyde (irinotecan liposome injection) along with 5-fluorouracil/leucovorin and oxaliplatin (NALIRIFOX regimen).
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content