US FDA grants Orphan Drug status to Avacta’s drug for soft tissue sarcoma
Pharmaceutical Technology
SEPTEMBER 6, 2022
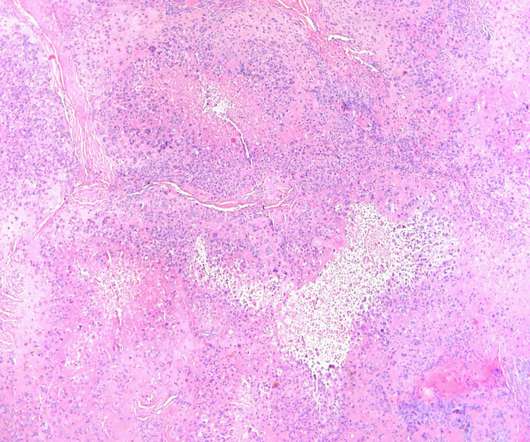
The US Food and Drug Administration (FDA) has awarded Orphan Drug Designation (ODD) to Avacta Group’s lead pre|CISION drug candidate, AVA6000, to treat soft tissue sarcoma. It also boosts the drug’s safety, tolerability and efficacy.




































Let's personalize your content