Key Developments from AMCP 2023 on Blockbuster Generics, Diabetes Drugs, PBM Legislation, and More
PM360
SEPTEMBER 21, 2023
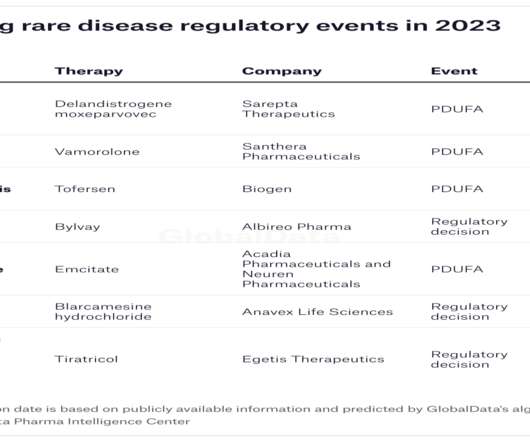
Jeff Casberg, MS, RPh, Senior Vice President of Clinical Pharmacy at IPD Analytics, a managed care and pharmaceutical consultancy, highlighted a wide range of 2023 key events that will affect managed care professionals by the end of the year. This product is expected to have three generic launches in 2023.
































Let's personalize your content