Intercept's latest NASH bid in jeopardy after FDA questions drug's efficacy, safety
Fierce Pharma
MAY 17, 2023
Intercept's latest NASH bid in jeopardy after FDA questions drug's efficacy, safety aliu Wed, 05/17/2023 - 12:29
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Fierce Pharma
MAY 17, 2023
Intercept's latest NASH bid in jeopardy after FDA questions drug's efficacy, safety aliu Wed, 05/17/2023 - 12:29

MedCity News
NOVEMBER 30, 2022
While an accelerated approval decision is expected in early 2023, the latest trial data are key because they represent the confirmatory study that could support an application for full FDA approval.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

pharmaphorum
NOVEMBER 28, 2023
FDA launches wide probe into CAR-T therapy safety Phil.Taylor Tue, 28/11/2023 - 18:27 Bookmark this

PharmaTech
SEPTEMBER 19, 2023
Maik Jornitz, Principal Consultant, BioProcess Resources LLC, discusses the definition of patient safety and how to implement new technologies into upgraded facilities.

pharmaphorum
JANUARY 6, 2023
From enabling patient choice during clinical trials to strengthening vital partnerships across the quality ecosystem, connected data will become the lifeblood that enables life sciences teams to collaborate efficiently and effectively in 2023. Safety will create a single source of truth for content management.

European Pharmaceutical Review
MARCH 27, 2024
percent from 2023 to 2032, according to the research. For example, in 2023, Lonza Group launched several new solutions for endotoxin and pyrogen testing, the report highlighted. However, due to the importance of pyrogen testing for the safety of pharmaceutical products , the report stated that this will help to drive market growth.

European Pharmaceutical Review
JANUARY 10, 2023
Clarivate Plc has released its Drugs to Watch 2023 report — among 70 of the drugs highlighted, including potential blockbuster drugs, the majority were revealed to be personalised medicines. The report offers predictive analysis of drugs entering the market or launching key indications in 2023.
European Pharmaceutical Review
OCTOBER 18, 2023
The US Food and Drug Administration (FDA) has approved XPHOZAH ® (tenapanor), the first and only phosphate absorption inhibitor. XPHOZAH is expected to be available to eligible patients in the US in November 2023. The post First-in-class phosphate absorption inhibitor approved by FDA appeared first on European Pharmaceutical Review.

European Pharmaceutical Review
NOVEMBER 6, 2023
The Phase III data suggests that] IZERVAY is an effective and safe treatment option for patients with geographic atrophy” “The safety profile over two years was consistent with year 1, with no new safety signals identified. Astellas to accelerate ocular disease treatments with $5.9

European Pharmaceutical Review
JANUARY 20, 2023
The US Food and Drug Administration (FDA) has issued a complete response letter for the accelerated approval submission of donanemab for early Alzheimer’s, Eli Lilly and Company revealed. The safety profile of donanemab was initially reported from the TRAILBLAZER-ALZ trial in the New England Journal of Medicine.

European Pharmaceutical Review
JANUARY 25, 2024
Draft guidance published by the US Food and Drug Administration (FDA) in December 2023, discussed quality considerations for topical ophthalmic drug products, including key considerations for extractables and leachables (E&L) testing. This document is revised from a version published in October 2023.

European Pharmaceutical Review
JUNE 26, 2023
US Food and Drug Administration (FDA) has published its first draft guidance presenting considerations to the pharmaceutical industry for designing clinical trials for psychedelic drugs. FDA’s draft guidance refers to psychedelics as “classic psychedelics”. These should be submitted latest 23 Aug 2023 to be considered.

Pharmaceutical Technology
APRIL 17, 2023
On 14 April 2023, experts from the US Food and Drug Administration’s (FDA) Advisory Committee (AdCom) voted largely in favour of the potential approval of Otsuka’ s and Lundbeck Pharmaceuticals’ Rexulti for the treatment of agitation associated with Alzheimer’s dementia (AAD). Rexulti is an atypical antipsychotic.

pharmaphorum
DECEMBER 22, 2022
Prediction 1: the FDA will become more directive and collaborative on guidelines for DCTs. The risk of not having these discussions is to conduct expensive trials only to discover too late that they were measuring the wrong endpoint, in the wrong way, or with a tool or technology the FDA does not accept.
Pharmaceutical Technology
APRIL 19, 2023
SAB Biotherapeutics has received breakthrough therapy designation (BTD) from the US Food and Drug Administration (FDA) for SAB-176, an investigational immunotherapy to treat influenza. SAB-176 received fast-track designation from FDA in mid-April 2023.

Pharmaceutical Technology
MARCH 2, 2023
The US Food and Drug Administration (FDA) has approved Reata Pharmaceuticals ’ oral, once-daily medication SKYCLARYS (omaveloxolone) to treat Friedreich’s ataxia patients. There are three more drug candidates with major trial readouts that are expected in 2023.

PharmaTech
FEBRUARY 21, 2024
FDA's Drug Safety Priorities FY23 describes the center’s key safety programs and activities involved in promoting and protecting public health.

Pharmaceutical Technology
JUNE 19, 2023
UK-based pharmaceutical giant GSK has announced that the US Food and Drug Administration (FDA) has extended the review period of its new drug application (NDA) for the rare bone cancer drug momelotinib by three months. Novartis and Incyte Corp’s Jakafi was the first FDA-approved drug for the treatment of myelofibrosis in November 2011.
Pharmaceutical Technology
JUNE 9, 2023
Biopharmaceutical company Novaliq has received approval from the US Food and Drug Administration (FDA) for VEVYE (cyclosporine ophthalmic solution) 0.1% Novaliq medical science and regulatory affairs vice-president Sonja Krösser stated: “We are proud that the FDA approved VEVYE. to treat the signs and symptoms of dry eye disease.
European Pharmaceutical Review
FEBRUARY 7, 2023
The US Food and Drug Administration (FDA) has granted Regenerative Medicine Advanced Therapy (RMAT) designation to RP-A501, an adeno-associated virus (AAV)-based gene therapy for Danon disease. “RP-A501 RMAT designation will provide the benefits of added intensive FDA guidance and expedited review through the programme’s development.
Pharmaceutical Technology
MAY 2, 2023
The US Food and Drug Administration (FDA) has accepted the supplemental biologics licence application submitted by Bristol Myers Squibb for Reblozyl (luspatercept-aamt) as a first-line treatment of anaemia in adults with lower-risk myelodysplastic syndromes (MDS).

European Pharmaceutical Review
MARCH 13, 2024
FDA inspections Identification of data integrity deviations Of the 70 Warning Letters issued by the US Food and Drug Administration (FDA) so far in 2024, three have identified data integrity issues at pharmaceutical manufacturing sites outside the US.

pharmaphorum
SEPTEMBER 30, 2022
Sarepta Therapeutics has followed through on its promise to file for accelerated approval of its gene therapy SRP-9001 for Duchenne muscular dystrophy (DMD), as it aims for a launch in the middle of 2023. The post Sarepta files Duchenne muscular dystrophy gene therapy with FDA appeared first on. Photo by Markus Spiske on Unsplash.

PM360
SEPTEMBER 21, 2023
Jeff Casberg, MS, RPh, Senior Vice President of Clinical Pharmacy at IPD Analytics, a managed care and pharmaceutical consultancy, highlighted a wide range of 2023 key events that will affect managed care professionals by the end of the year. This product is expected to have three generic launches in 2023.

Pharmaceutical Technology
JUNE 1, 2023
The US Food and Drug Administration (FDA) has granted a combination of AstraZeneca and MSD ’s Lynparza (olaparib), with standard therapies for treating BRCA-mutated (BRCAm) metastatic castration-resistant prostate cancer (mCRPC). Safety and tolerability were in line with that observed in prior trials and the known profiles of the medicines.
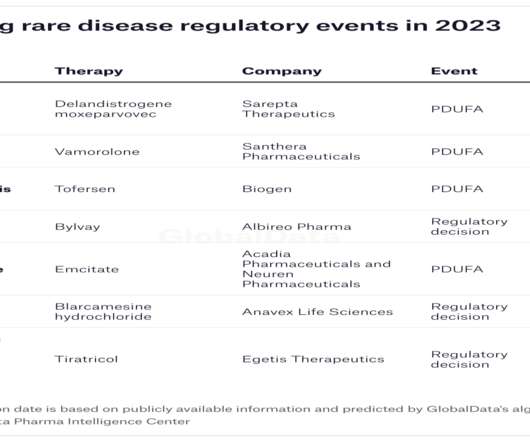
Pharmaceutical Technology
JANUARY 22, 2023
In 2022, the FDA approved only 37 new medicines, an underwhelming number compared to 98 in 2018. More therapies to meet unmet needs GlobalData predicts that at least 35 US regulatory decisions on drugs for rare diseases are on the horizon for 2023. GlobalData is the parent company of Pharmaceutical Technology.
European Pharmaceutical Review
FEBRUARY 21, 2023
SYFOVRE (pegcetacoplan injection) is the first and only treatment approved by the US Food and Drug Administration (FDA) for geographic atrophy (GA), a leading cause of blindness. The safety profile of SYFOVRE is well-demonstrated following ~12,000 injections. SYFOVRE is expected to be available by the beginning of March 2023.

European Pharmaceutical Review
JUNE 27, 2023
AC Immune SA has received Fast Track designation from the US Food and Drug Administration (FDA) for its anti-amyloid beta (Abeta) active immunotherapy vaccine candidate for Alzheimer’s disease. The anti-Abeta therapy “specifically targets the most toxic forms of Abeta” according to Dr Andrea Pfeifer, CEO of AC Immune SA.

pharmaphorum
AUGUST 1, 2022
Sarepta is pressing forward with a bold plan to file with the FDA for accelerated approval of its gene therapy SRP-9001 for Duchenne muscular dystrophy (DMD) in the next few months, with a view to making it available in sometime around the middle of 2023.

Legacy MEDSearch
JANUARY 13, 2023
implantation of the HIT Reverse Hip Replacement System (Reverse HRS), under an FDA approved Investigational Device Exemption (IDE). The IDE Study is being conducted to determine the safety and effectiveness of the HIT Reverse HRS in Primary Total Hip Arthroplasty (THA). Press Release by: Hip Innovation Technology. Are you hiring?

European Pharmaceutical Review
NOVEMBER 19, 2024
This new collaboration is set to further improve safety and efficacy of radiopharmaceuticals. The US Food and Drug Administration (FDA) approved Novartis’ Pluvicto (lutetiumlutetium Lu 177 vipivotide tetraxetan) in 2022. “Radioligand therapies hold transformative potential for certain forms of cancer.”
European Pharmaceutical Review
JUNE 29, 2023
Initiating Phase II trials for an AI-generated drug The Phase II study will assess the safety, tolerability, pharmacokinetics and preliminary efficacy of 12-week oral INS018_055 dosage in subjects with the rare lung disease idiopathic pulmonary fibrosis (IPF). In early 2023, INS018_055 received positive topline data in Phase I.

Pharmatutor
FEBRUARY 2, 2023
FDA Inspections - Overview admin Thu, 02/02/2023 - 13:52 FDA Inspection is a regulatory process conducted by the United States Food and Drug Administration (FDA) to evaluate the compliance of food and drug establishments with FDA regulations and standards.
European Pharmaceutical Review
JANUARY 5, 2023
The US Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) has accepted the Biologics License Application (BLA) for nirsevimab for the prevention of respiratory syncytial virus (RSV) lower respiratory tract infection (LRTI) in all infants. The safety profile of nirsevimab was similar to placebo.

European Pharmaceutical Review
JANUARY 23, 2025
billion, the agreement is the biggest biotech M&A transaction since 2023. For example, 2024 saw companies trying to tap innovation at an earlier point in the development cycle, and the industry turned away from the big deals for de-risked assets that characterised 2023. FDA Issues First Recommendations On AI For Drug Development.
European Pharmaceutical Review
AUGUST 22, 2023
The US Food and Drug Administration (FDA)-approved treatment, is the first RSV vaccine indicated for infants from birth to six months of age. US FDA granted approval of Abrysvo to Pfizer Inc. This news follows similar regulatory action by the US FDA in recent months. percent within 90 days after birth.
European Pharmaceutical Review
SEPTEMBER 18, 2023
The first and only treatment for anaemic patients with myelofibrosis has been approved by the US Food and Drug Administration (FDA). Clinical evidence for Ojjaara Approval of momelotinib by the US FDA is supported by data from the Phase III MOMENTUM trial and a subpopulation of adult patients with anaemia from the SIMPLIFY-1 Phase III trial.
European Pharmaceutical Review
OCTOBER 24, 2023
PENBRAYA (meningococcal groups A, B, C, W and Y vaccine), the first and only pentavalent vaccine that provides the broadest serogroup coverage of any meningococcal vaccine available in the US for meningococcal disease in individuals aged 10 to 25 years old, has been approved by the US Food and Drug Administration (FDA). About PENBRAYA?

European Pharmaceutical Review
FEBRUARY 2, 2024
Biogen’s decision A strategic review of the company’s R&D work evaluated the time and investment required for the post-marketing confirmatory ENVISION study and the likely advancements in the field by the time of potential ADUHELM FDA traditional approval, Biogen explained.
Pharmaceutical Technology
FEBRUARY 26, 2023
This is largely due to the FDA’s rigorous approach to the safety of microbiome therapeutics, which has manifested in clinical holds, resulting in delays that have dimmed the enthusiasm in the space in recent times. While the French MaaT Pharma has submitted further information to the FDA, its trial remains on hold. with placebo.

European Pharmaceutical Review
OCTOBER 30, 2023
Moreover, no unexpected safety signal for the gene therapy was identified in the LGMD2I/R9 study. Additionally, these first results were also presented at the 2023 International Limb-Girdle Muscular Dystrophy Conference. ATA-100 has received an Investigational New Drug (IND) clearance by the US Food and Drug Administration (FDA).

European Pharmaceutical Review
NOVEMBER 29, 2023
Following the US Food and Drug Administration (FDA)’s approval of Ferring Pharmaceuticals’ gene therapy Adstiladrin ® (nadofaragene firadenovec-vncg) in December 2022, new long-term follow up data has been revealed. These findings were presented at the 2023 Annual Meeting of the Society of Urologic Oncology (SUO).

European Pharmaceutical Review
AUGUST 31, 2023
Overcoming challenges in patient safety, manufacturing and supply of radiopharmaceuticals As clinical trials progress and the first results are published, companies’ best candidates will emerge in the next five years. Hospital staff must also be protected from radiation while handling the agent.

European Pharmaceutical Review
JUNE 30, 2023
Roctavian, an adeno-associated virus (AAV) vector-based gene therapy is the first to be approved by the US Food and Drug Administration (FDA) for adults with severe haemophilia A. The gene therapy is authorised for individuals without pre-existing antibodies to adeno-associated virus serotype 5 detected by an FDA-approved test.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content