Shareholders versus patients: Who is more important?
World of DTC Marketing
JUNE 22, 2021
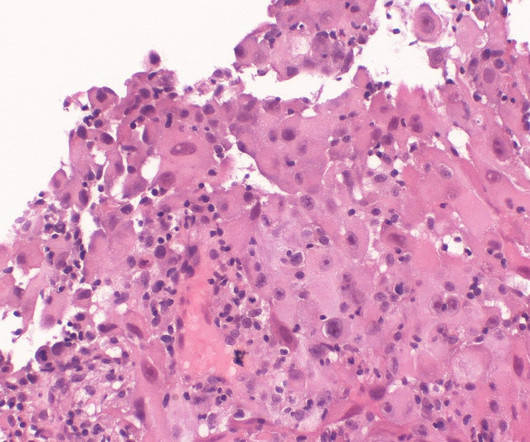
Milton Friedman A newly approved drug to treat Alzheimer’s disease is expected to become a multibillion-dollar expense for Medicare. By one projection, spending on the drug for Medicare patients could end up being higher than the budgets for the Environmental Protection Agency or NASA. Milton Friedman.




























Let's personalize your content