Paving the way for anti-Abeta active immunotherapy
European Pharmaceutical Review
SEPTEMBER 22, 2023
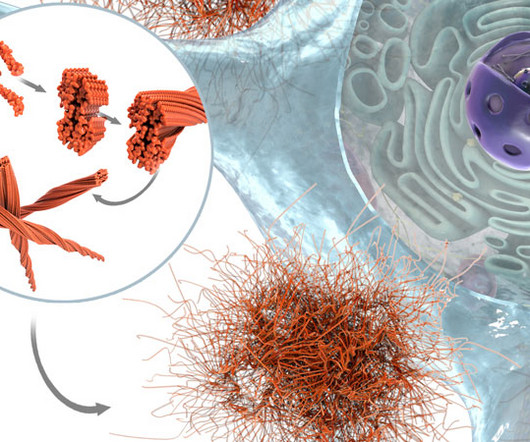
7 It is currently being evaluated in a Phase Ib/IIa clinical study, demonstrating to date a good safety profile. While favourable safety has been reported up to the Phase II, the relevance of targeting the C-terminal portion of Abeta still needs to be confirmed.








Let's personalize your content