The rise of prescription drug abuse and drug diversion
pharmaphorum
JANUARY 12, 2023
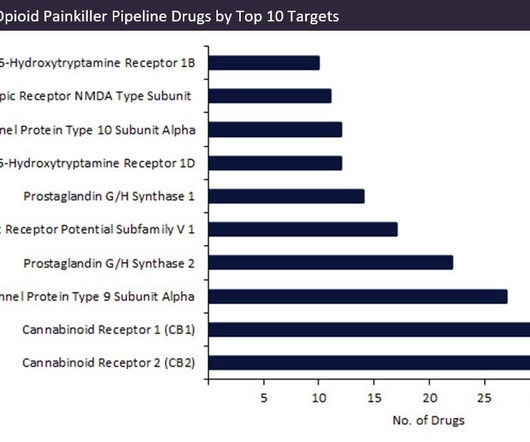
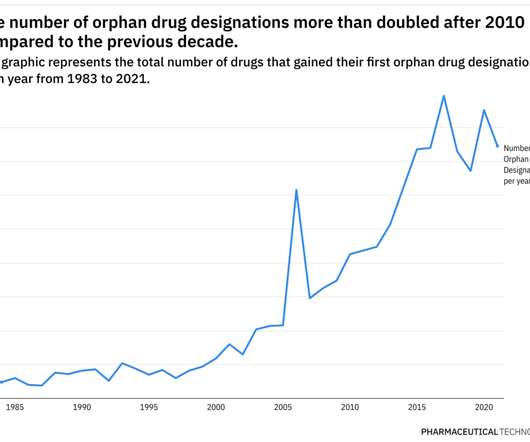
As prescription drug abuse has grown in the US and Europe, the diversion of such treatments from legitimate sources has also increased. Drug diversion is the channelling of prescription drugs from a medical source into the illegal market.



















Let's personalize your content