Biogen’s $900 million settlement signals scrutiny on speaker fees
Pharmaceutical Technology
OCTOBER 5, 2022
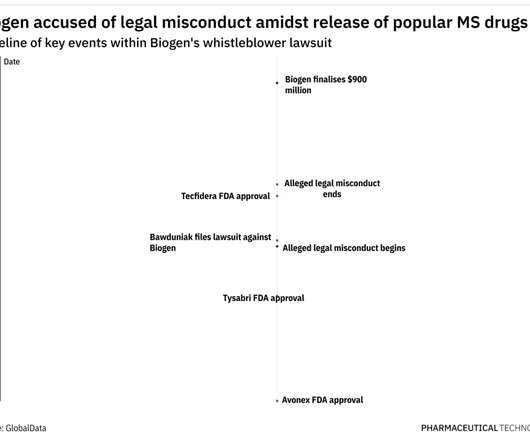
In 2009, Tysabri was performing well, having yielded $776 million in sales after being first approved in 2004, as per Biogen’s 2009 financial filings. However, new MS drugs like Novartis ’ Gilenya, were going to soon enter the market and increase competition within the MS therapeutic landscape.











Let's personalize your content