The State of the Union in Cancer Innovation
PM360
JULY 13, 2023
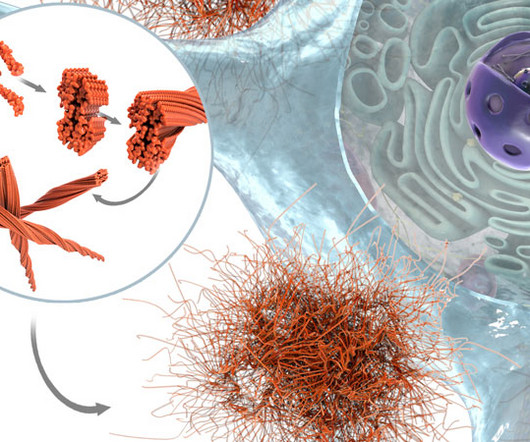
The FDA has granted Fast Track Designation for our HER2-targeted ADC, ARX788, and we are enrolling the signal-seeking ACE-Breast-03 Phase 2 clinical study in post-Enhertu mBC patients throughout 2H 2023. Not only can we design drugs better, but we can understand their effects better to engineer small molecules in a more efficient way.









Let's personalize your content